|
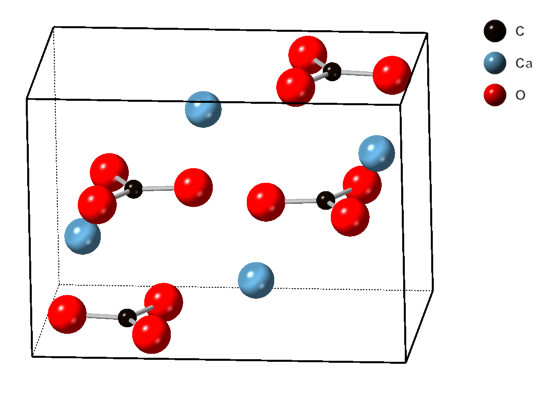
Aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation from marine and freshwater environments. The crystal lattice of aragonite differs from that of calcite, resulting in a different crystal shape, an orthorhombic crystal system with acicular crystal. Repeated twinning results in pseudo-hexagonal forms. Aragonite may be columnar or fibrous, occasionally in branching helictitic forms called ''flos-ferri'' ("flowers of iron") from their association with the ores at the Carinthian iron mines. Occurrence The type location for aragonite is Molina de Aragón in the Province of Guadalajara in Castilla-La Mancha, Spain, for which it was named in 1797. Aragonite is found in this locality as cyclic twins inside gypsum and marls of the Keuper facies of the Triassic. This type of aragoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues. Chemistry Calcium carbonate shares the typical properties of other carbonates. Notably, it: *reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water: : *releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above 840 °C in the case of ), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Mineral
Carbonate minerals are those minerals containing the carbonate ion, . Carbonate divisions Anhydrous carbonates *Calcite group: trigonal **Calcite CaCO3 **Gaspéite (Ni,Mg,Fe2+)CO3 **Magnesite MgCO3 **Otavite CdCO3 **Rhodochrosite MnCO3 **Siderite FeCO3 **Smithsonite ZnCO3 **Spherocobaltite CoCO3 *Aragonite group: orthorhombic **Aragonite CaCO3 **Cerussite PbCO3 **Strontianite SrCO3 **Witherite BaCO3 **Rutherfordine UO2CO3 **Natrite Na2CO3 Anhydrous carbonates with compound formulas *Dolomite group: trigonal **Ankerite CaFe(CO3)2 **Dolomite (mineral), Dolomite CaMg(CO3)2 **Huntite Mg3Ca(CO3)4 **Minrecordite CaZn(CO3)2 **Barytocalcite BaCa(CO3)2 Carbonates with hydroxyl or halogen *Carbonate with hydroxide: monoclinic **Azurite Cu3(CO3)2(OH)2 **Hydrocerussite Pb3(CO3)2(OH)2 **Malachite Cu2CO3(OH)2 **Rosasite (Cu,Zn)2CO3(OH)2 **Phosgenite Pb2(CO3)Cl2 **Hydrozincite Zn5(CO3)2(OH)6 **Aurichalcite (Zn,Cu)5(CO3)2(OH)6 Hydrated carbonates *Hydromagnesite Mg5(CO3)4(OH)2.4H2O *Ikaite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on Scratch hardness, scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German , a term from the 19th century that came from the Latin word for Lime (material), lime, (genitive ) with the suffix ''-ite'' used to name minerals. It is thus a Doublet (linguistics), doublet of the word ''wikt:chalk, chalk''. When applied by archaeology, archaeologists and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In crystallography, polymorphism is the phenomenon where a compound or element can crystallize into more than one crystal structure. The preceding definition has evolved over many years and is still under discussion today. Discussion of the defining characteristics of polymorphism involves distinguishing among types of transitions and structural changes occurring in polymorphism versus those in other phenomena. Overview Phase transitions (phase changes) that help describe polymorphism include polymorphic transitions as well as melting and vaporization transitions. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." Additionally, Walter McCrone described the phases in polymorphic matter as "different in crystal structure but identical in the liquid or vapor states." McCrone also def ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Twinning
Crystal twinning occurs when two or more adjacent crystals of the same mineral are oriented so that they share some of the same crystal lattice points in a symmetrical manner. The result is an intergrowth of two separate crystals that are tightly bonded to each other. The surface along which the lattice points are shared in twinned crystals is called a composition surface or twin plane. Crystallographers classify twinned crystals by a number of twin laws, which are specific to the crystal structure. The type of twinning can be a diagnostic tool in mineral identification. There are three main types of twinning. The first is growth twinning which can occur both in very large and very small particles. The second is transformation twinning, where there is a change in the crystal structure. The third is deformation twinning, in which twinning develops in a crystal in response to a shear stress, and is an important mechanism for permanent shape changes in a crystal. Definition T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type Locality (geology)
Type locality, also called type area, is the locality where a particular rock type, stratigraphic unit or mineral species is first identified. If the stratigraphic unit in a locality is layered, it is called a stratotype, whereas the standard of reference for unlayered rocks is the type locality. The concept is similar to type site in archaeology. Examples of geological type localities Rocks and minerals * Aragonite: Molina de Aragón, Guadalajara, Spain * Autunite: Autun, France * Benmoreite: Ben More (Mull), Scotland * Blairmorite: Blairmore, Alberta, Canada * Boninite: Bonin Islands, Japan * Comendite: Comende, San Pietro Island, Sardinia * Cummingtonite: Cummington, Massachusetts * Dunite: Dun Mountain, New Zealand * Essexite: Essex County, Massachusetts, US * Fayalite: Horta, Fayal Island, Azores, Portugal * Harzburgite: Bad Harzburg, Germany * Icelandite: Thingmuli (Þingmúli), Iceland * Ijolite: Iivaara, Kuusamo, Finland * Kimberlite: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helictite
A helictite is a speleothem (cave-formed mineral) found in a Solution cave, limestone cave that changes its axis from the vertical at one or more stages during its growth. Helictites have a curving or angular form that looks as if they were grown in zero gravity. They are most likely the result of Capillary action, capillary forces acting on tiny water droplets, a force often strong enough at this scale to defy gravity. Helictites are, perhaps, the most delicate of cave formations. They are usually made of needle-form calcite and aragonite. Helictite forms have been described in several types: ribbon helictites, saws, rods, butterflies, "hands", curly-fries, and "clumps of worms". They typically have symmetry (biology)#Radial symmetry, radial symmetry. They can be easily crushed or broken by the slightest touch. Because of this, helictites are rarely seen within arm's reach in Tourism, tourist caves. Timpanogos Cave National Monument in Utah has one of the largest collections o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic Crystal System
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal In mathematics, orthogonality (mathematics), orthogonality is the generalization of the geometric notion of ''perpendicularity''. Although many authors use the two terms ''perpendicular'' and ''orthogonal'' interchangeably, the term ''perpendic .... Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Duchy Of Carinthia
The Duchy of Carinthia (; ; ) was a duchy located in southern Austria and parts of northern Slovenia. It was separated from the Duchy of Bavaria in 976, and was the first newly created Imperial State after the original German stem duchies. Carinthia remained a State of the Holy Roman Empire until its dissolution in 1806, though from 1335 it was ruled within the Austrian dominions of the Habsburg dynasty. A constituent part of the Habsburg monarchy and of the Austrian Empire, it remained a Cisleithanian crown land of Austria-Hungary until 1918. By the 1920 Carinthian plebiscite in October 1920, the main area of the duchy formed the Austrian state of Carinthia. History In the seventh century the area was part of the Slavic principality of Carantania, which fell under the suzerainty of Duke Odilo of Bavaria in about 743. The Bavarian stem duchy was incorporated into the Carolingian Empire when Charlemagne deposed Odilo's son Duke Tassilo III in 788. In the 843 partition b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freshwater Ecosystem
Freshwater ecosystems are a subset of Earth's aquatic ecosystems that include the biological communities inhabiting freshwater waterbodies such as lakes, ponds, rivers, streams, springs, bogs, and wetlands. They can be contrasted with marine ecosystems, which have a much higher salinity. Freshwater habitats can be classified by different factors, including temperature, light penetration, nutrients, and vegetation. There are three basic types of freshwater ecosystems: lentic (slow moving water, including pools, ponds, and lakes), lotic (faster moving streams, for example creeks and rivers) and wetlands ( semi-aquatic areas where the soil is saturated or inundated for at least part of the time). Freshwater ecosystems contain 41% of the world's known fish species. Freshwater ecosystems have undergone substantial transformations over time, which has impacted various characteristics of the ecosystems. Original attempts to understand and monitor freshwater ecosystems were spurre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |