|
Zincate
In chemistry the term zincate may refer to several substances containing the element zinc: * usually the anion Zn(OH)42−, more properly called tetrahydroxozincate or salt (chemistry), salts thereof, such as sodium zincate . * the polymeric anion [Zn(OH)3−] and its salts, for example NaZn(OH)3· H2O. * an oxide containing zinc and a less electronegativity, electronegative element e.g. Na2ZnO2. In the health supplement industry zincate may also mean a commercially available zinc supplement, typically formulated as zinc sulfate. Solution (chemistry), Solutions prepared from dissolving zinc hydroxide or zinc oxide in a strong alkali like sodium hydroxide, which contains various zincate anions, are used in the metal plating industry, in processes such as immersion zinc plating and electroplating (electrogalvanization). Any of these techniques may be called zincate process. Inorganic compound nomenclature In the IUPAC nomenclature of inorganic chemistry, naming of inorganic comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydroxozincate
In chemistry, tetrahydroxozincate or tetrahydroxidozincate is a valence (chemistry), divalent anion (negative ion) with formula , with a central zinc atom in the +2 or (II) valence state coordination compound, coordinated to four hydroxide groups. It has Sp3 hybridization. It is the most common of the zincate anions, and is often called just zincate. These names are also used for the salts containing that anion, such as sodium zincate Na2Zn(OH)4 and calcium zincate CaZn(OH)4·2H2O Zincate salts can be obtained by reaction of zinc oxide (ZnO) or zinc hydroxide () and a strong base (chemistry), base like sodium hydroxide. It is now generally accepted that the resulting solutions contain the tetrahydroxozincate ion. Earlier Raman studies had been interpreted as indicating the existence of linear ions. Related anions and salts The name "zincate" may also refer to a polymeric anion with formula approaching []''n'', which forms salts such as ·, or to mixed oxides of zinc and less ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
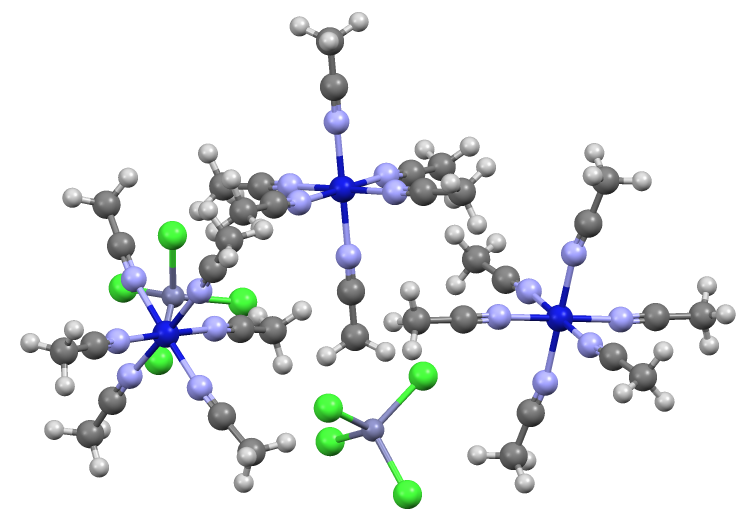
Tetrachlorozincate
Tetrachlorozincate is an anion with the formula . It is a counterion that is often used in conjunction with strong electrophiles. Being dianionic, tetrachlorozincate is not classified as a weakly coordinating anion. On the other hand, being dianionic, tetrachlorozincate facilitates the crystallization of many salts. It has a tetrahedral molecular geometry. A simple example is (ammonium tetrachlorozincate). Zincates are anionic zinc complexes. Related to the preparation of Lucas' reagent, tetrachlorozincates are often generated by combining hydrochloric acid and zinc chloride. A related anion is , in which again Zn(II) adopts a tetrahedral geometry. References {{Reflist Tetrachlorozincates, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Zincate
Sodium zincate refers to anionic zinc oxides or hydroxides, depending on conditions. In the applications of these materials, the exact formula is not necessarily important and it is likely that aqueous zincate solutions consist of mixtures. Hydroxyzincates Solutions of sodium zincate may be prepared by dissolving zinc, zinc hydroxide, or zinc oxide in an aqueous solution of sodium hydroxide Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly corrosive base (chemistry), .... Simplified equations for these complex processes are: :ZnO + H2O + 2 NaOH → Na2Zn(OH)4 :Zn + 2 H2O + 2 NaOH → Na2Zn(OH)4 + H2 From such solutions, one can crystallize salts of containing the anions Zn(OH)42−, Zn2(OH)62−, and Zn(OH)64−. Na2Zn(OH)4 consists of tetrahedral zincate ion and octahedral sodium cations. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immersion Zinc Plating
Immersion zinc plating is an electroless (non-electrolytic) coating process that deposits a thin layer of zinc on a less electronegative metal, by immersion in a solution containing a zinc or zincate ions, . A typical use is plating aluminum with zinc prior to electrolytic or electroless nickel plating. Immersion zinc plating involves the displacement of zinc from zincate by the underlying metal: :3 + 2 Al → 3 Zn + 2 + 4 OH− See also * Electrogalvanization Electrogalvanizing is a process in which a layer of zinc is bonded to steel to protect against corrosion, enhance adhesion, or give an aesthetic appeal. The process involves electroplating, running a current of electricity through a saline-/zinc-ba ... (electrolytic zinc coating) * Immersion gold plating * Immersion copper plating * Immersion silver plating References Glenn O. Mallory, Juan B. Hajdu (1990), Electroless Plating: Fundamentals and Applications, American Electroplaters and Surface Finishers Society, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It is the second most abundant trace metal in humans after iron, an import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Compounds
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 element, group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript appearance and behavior: they are generally colorless (unlike compounds of other elements with oxidation number +2, which are colored), do not readily engage in redox reactions, and generally adopt symmetrical structures. General characteristics In its compounds, Zn2+ ions have an electronic configuration [Ar] 3d10. As such, Zn2+ tends to have a symmetrical coordination geometry in both its complexes and compounds. In both ZnO and ZnS, (zincblende) zinc is bound tetrahedrally bound to four ligands (oxide and sulfide, respectively). Many coordination complex, complexes, such as ZnCl42−, are tetrahedral. Tetrahedrally coordinated zin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrogalvanization
Electrogalvanizing is a process in which a layer of zinc is bonded to steel to protect against corrosion, enhance adhesion, or give an aesthetic appeal. The process involves electroplating, running a current of electricity through a saline-/zinc-based electrolytic solution with a zinc anode and steel cathode. Such zinc electroplating or zinc alloy electroplating maintains a dominant position among other electroplating process options, based upon electroplated tonnage per annum. According to the International Zinc Association, more than 5 million tons are used yearly for both hot-dip galvanization and electroplating. The plating of zinc was developed at the beginning of the 20th century. At that time, the electrolytic solution was cyanide-based. A significant innovation occurred in the 1960s with the introduction of the first acid chloride-based electrolyte. The 1980s saw a return to alkaline electrolytes, only this time, without the use of cyanide. The most commonly used electrogalva ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ate Complex
In chemistry, an ate complex is a salt formed by the reaction of a Lewis acid with a Lewis base whereby the central atom (from the Lewis acid) increases its valence and gains a negative formal charge. (In this definition, the meaning of valence is equivalent to coordination number). Often in chemical nomenclature the term ''ate'' is suffixed to the element in question. For example, the ate complex of a boron compound is called a borate. Thus trimethylborane and methyllithium react to form the ate compound , lithium tetramethylborate(1-). This concept was introduced by Georg Wittig in 1958. Ate complexes are common for metals, including the transition metals (groups 3-11), as well as the metallic or semi-metallic elements of group 2, 12, and 13. They are also well-established for third-period or heavier elements of groups 14–18 in their higher oxidation states. Ate complexes are a counterpart to onium ions. Lewis acids form ate ions when the central atom reacts with a donor ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Hydroxide
Zinc hydroxide Zn( OH)2 is an inorganic chemical compound. It also occurs naturally as 3 rare minerals: wülfingite (orthorhombic), ashoverite and sweetite (both tetragonal). Like the hydroxides of other metals, such as lead, aluminium, beryllium, tin and chromium, Zinc hydroxide (and Zinc oxide), is amphoteric. Thus it will dissolve readily in a dilute solution of a strong acid, such as HCl, and also in a solution of an alkali such as sodium hydroxide. Preparation It can be prepared by first dissolving zinc oxide in concentrated aqueous solution of sodium hydroxide. The resulting solution is strongly diluted. :Zn2+ + 2 OH− → Zn(OH)2. The initial colorless solution contains the zincate ion: :Zn(OH)2 + 2 OH− → Zn(OH)42−. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; when excess sodium hydroxide is added to the solution the hydroxide ions will reduce the complex to a −2 charge and make it soluble. When excess ammonia i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Oxide
Zinc oxide is an inorganic compound with the Chemical formula, formula . It is a white powder which is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, Zinc metabolism, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, sunscreens, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, semi conductors, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically. History Early humans probably used zinc compounds in processed and unprocessed forms, as paint or medicinal ointment; however, their composition is uncertain. The use of ''pushpanjan'', probably zinc oxide, as a salve for eyes and open wounds is mentioned in the Indian medical text the Charaka Samhita, thought to date from 500 BC or before. Zinc oxide ointment is also mentioned by the Greek physician Dioscorides (1st century AD). Galen suggested treatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly corrosive base (chemistry), base and alkali that decomposes lipids and proteins at ambient temperatures and at high concentrations may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the making of wood pulp and paper, tex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Plating
Plating is a finishing process in which a metal is deposited on a surface. Plating has been done for hundreds of years; it is also critical for modern technology. Plating is used to decorate objects, for corrosion inhibition, to improve solderability, to harden, to improve wearability, to reduce friction, to improve paint adhesion, to alter conductivity, to improve IR reflectivity, for radiation shielding, and for other purposes. Jewelry typically uses plating to give a silver or gold finish. Thin-film deposition has plated objects as small as an atom, therefore plating finds uses in nanotechnology. There are several plating methods, and many variations. In one method, a solid surface is covered with a metal sheet, and then heat and pressure are applied to fuse them (a version of this is Sheffield plate). Other plating techniques include electroplating, vapor deposition under vacuum and sputter deposition. Recently, plating often refers to using liquids. Metallizing refers to c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |