Zinc Compounds on:
[Wikipedia]
[Google]
[Amazon]
Zinc compounds are
 Zinc compounds, like those of
Zinc compounds, like those of
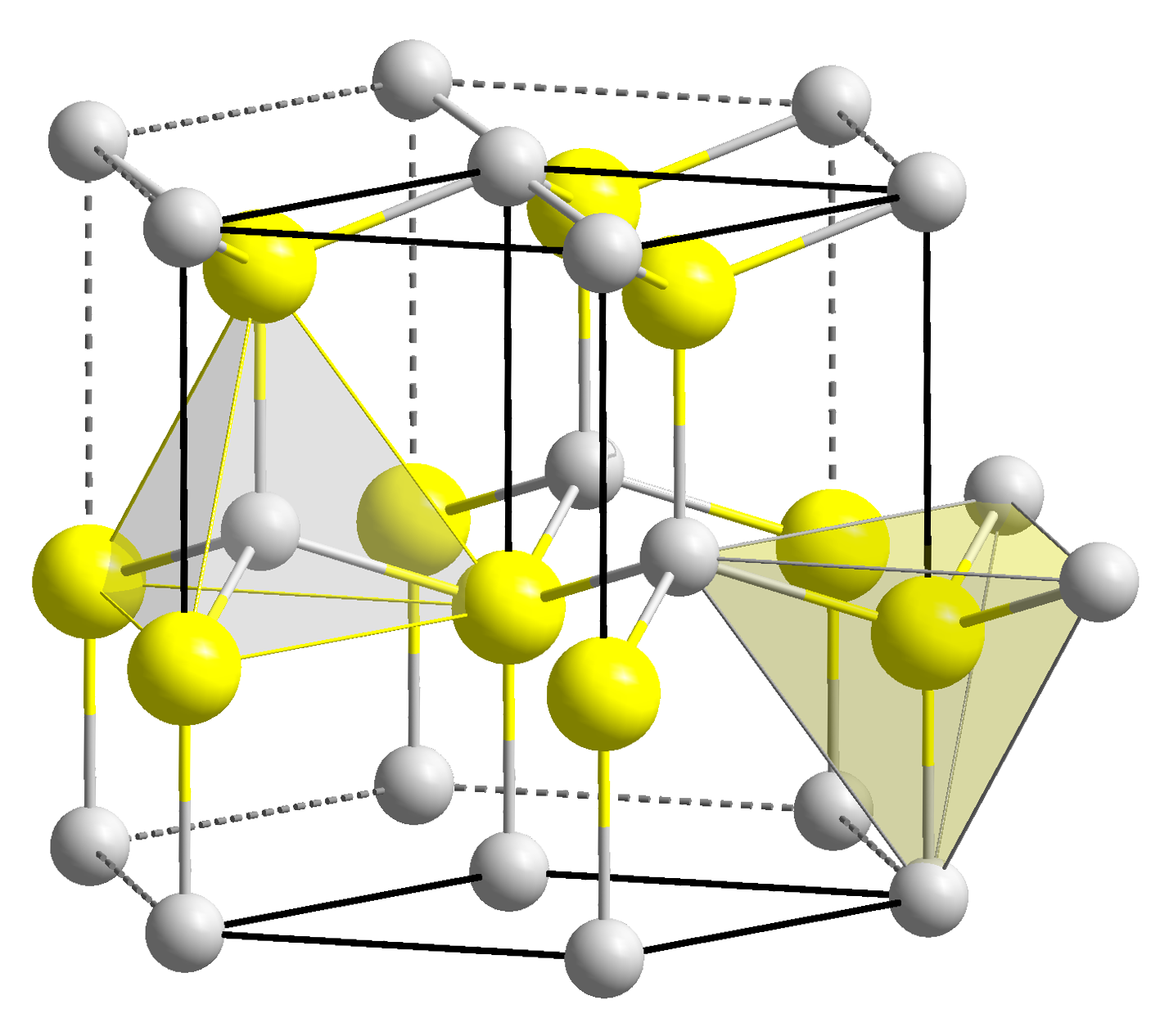
 Zinc oxide, ZnO, is the most important manufactured compound of zinc, with a wide variety of uses. It crystallizes with the Wurtzite structure. It is
Zinc oxide, ZnO, is the most important manufactured compound of zinc, with a wide variety of uses. It crystallizes with the Wurtzite structure. It is
koettigite
are a few examples of other common inorganic compounds of zinc. The latter two compounds are both used in insecticides and wood preservatives. One of the simplest examples of an
 ]
The most common structure of zinc complexes is tetrahedral. Nevertheless, octahedral complexes comparable to those of the earlier transition metals are not rare. Zn2+ is a stability constants of complexes#Classification of metal ions, class A acceptor in the classification of Ahrland, Chatt and Davies, and so forms stronger complexes with the first-row donor atoms oxygen or nitrogen than with second-row sulfur or phosphorus. In terms of
]
The most common structure of zinc complexes is tetrahedral. Nevertheless, octahedral complexes comparable to those of the earlier transition metals are not rare. Zn2+ is a stability constants of complexes#Classification of metal ions, class A acceptor in the classification of Ahrland, Chatt and Davies, and so forms stronger complexes with the first-row donor atoms oxygen or nitrogen than with second-row sulfur or phosphorus. In terms of

 A very large number of metallo-enzymes contain zinc(II). Also many
A very large number of metallo-enzymes contain zinc(II). Also many

chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s containing the element zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
which is a member of the group 12 of the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
. The oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element
In chemistry and atomic physics, the main group is the group (periodic table), group of chemical element, elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon ...
with zinc(II). Zinc compounds are noteworthy for their nondescript appearance and behavior: they are generally colorless (unlike compounds of other elements with oxidation number +2, which are colored), do not readily engage in redox reactions, and generally adopt symmetrical structures.
General characteristics
In its compounds, Zn2+ ions have anelectronic configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
r3d10. As such, Zn2+ tends to have a symmetrical coordination geometry in both its complexes and compounds. In both ZnO and ZnS, (zincblende
Sphalerite is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-Valley type, and volcanogenic mas ...
) zinc is bound tetrahedrally bound to four ligands (oxide and sulfide, respectively). Many complexes, such as ZnCl42−, are tetrahedral. Tetrahedrally coordinated zinc is found in metallo-enzymes such as carbonic anhydrase
The carbonic anhydrases (or carbonate dehydratases) () form a family of enzymes that catalyst, catalyze the interconversion between carbon dioxide and water and the Dissociation (chemistry), dissociated ions of carbonic acid (i.e. bicarbonate a ...
. Six-coordinate octahedral complexes are also common, such as the aquo complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorat ...
n(H2O)6sup>2+, which is present when a zinc salts are dissolved in water. Five- and seven-coordination numbers can be imposed by special organic ligands.
Many zinc(II) salts are isomorphous (have the same type of crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
) with the corresponding salts of magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
(II). This parallel results from the fact that Zn2+ and Mg2+ have almost identical ionic radii
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation ...
as well as filled electron shells. That two elements so different in atomic number have the same radius is a consequence of the d-block contraction
The d-block contraction (sometimes called scandide contraction) is a term used in chemistry to describe the effect of having full d orbitals on the period 4 elements. The elements in question are gallium, germanium, arsenic, selenium, bromine ...
. Whilst calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
is somewhat larger than magnesium, there is a steady decrease in size as atomic number increases from calcium to zinc.
Zn(II) complexes are kinetically labile, i.e. the Zn-ligand bonds exchange with other ligands rapidly. For this reason, zinc ions are at the catalytic centers in many enzymes.
Zn(I)
Compounds with zinc in the oxidation state +1 are extremely rare. The compounds have the formula RZn2R and they contain a Zn — Zn bond analogous to the metal-metal bond in mercury(I) ion, Hg22+. In this respect zinc is similar to magnesium where low-valent compounds containing a Mg — Mg bond have been characterised.Other oxidation states
No compounds of zinc in oxidation states other than +1 or +2 are known. Calculations indicate that a zinc compound with the oxidation state of +4 is unlikely to exist.Colour and magnetism
 Zinc compounds, like those of
Zinc compounds, like those of main group element
In chemistry and atomic physics, the main group is the group (periodic table), group of chemical element, elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon ...
s, are mostly colourless. Exceptions occur when the compound contains a coloured anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
or ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
. However, zinc selenide
Zinc selenide is the inorganic compound with the formula ZnSe. It is a lemon-yellow solid although most samples have a duller color due to the effects of oxidation. It is an intrinsic semiconductor with a band gap of about 2.70 eV at , equi ...
and zinc telluride
Zinc telluride is a binary chemical compound with the formula ZnTe. This solid is a semiconductor material with a direct band gap of 2.26 eV. It is usually a p-type semiconductor. Its crystal structure is cubic, like that for sphalerite and diam ...
are both coloured due to charge-transfer processes. Zinc oxide turns yellow when heated due to the loss of some oxygen atoms and formation of a defect structure. Compounds containing zinc are typically diamagnetic
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagn ...
, except in cases where the ligand is a radical.
Reactivity of metallic zinc
Zinc is a strongreducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
with a standard redox potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respe ...
of −0.76 V. Pure zinc tarnishes rapidly in air, rapidly forming a passive layer. The composition of this layer can be complex, but one constituent is probably basic zinc carbonate, Zn5(OH)6CO3. The reaction of zinc with water is slowed by this passive layer. When this layer is corroded by acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
s such as hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
and sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, the reaction proceeds with the evolution of hydrogen gas.
:Zn + 2 H+ → Zn2+ + H2
Zinc reacts with alkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The a ...
s as with acids.
With oxidants such as chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
s and halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s, Zn forms binary compounds such as ZnS and ZnCl2.
Binary compounds

 Zinc oxide, ZnO, is the most important manufactured compound of zinc, with a wide variety of uses. It crystallizes with the Wurtzite structure. It is
Zinc oxide, ZnO, is the most important manufactured compound of zinc, with a wide variety of uses. It crystallizes with the Wurtzite structure. It is amphoteric
In chemistry, an amphoteric compound () is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.
Etymology and terminology
Amphoteric is d ...
, dissolving in acids to give the aqueous Zn2+ ion and in alkali to give the zincate
In chemistry the term zincate may refer to several substances containing the element zinc:
* usually the anion Zn(OH)42−, more properly called tetrahydroxozincate or salt (chemistry), salts thereof, such as sodium zincate .
* the polymeric anion ...
(a.k.a. tetrahydroxozincate) ion, n(OH)4sup>2−. Zinc hydroxide
Zinc hydroxide Zn( OH)2 is an inorganic chemical compound. It also occurs naturally as 3 rare minerals: wülfingite (orthorhombic), ashoverite and sweetite (both tetragonal).
Like the hydroxides of other metals, such as lead, aluminium, beryl ...
, Zn(OH)2 is also amphoteric.
Zinc sulfide, ZnS, crystallizes in two closely related structures, the zincblende crystal structure
Sphalerite is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-Valley type, and volcanogenic mas ...
and the Wurtzite crystal structure
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal ...
, which are common structures of compounds with the formula MA. Both Zn and S are tetrahedrally coordinated by the other ion. A useful property of ZnS is its phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluor ...
. The other chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
ides, ZnSe and ZnTe, have applications in electronics and optics.
Of the four zinc halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
s, has the most ionic character, whereas the others, , , and , have relatively low melting points and are considered to have more covalent character. The pnictogen
, -
! colspan=2 style="text-align:left;" , ↓ Period
, -
! 2
,
, -
! 3
,
, -
! 4
,
, -
! 5
,
, -
! 6
,
, -
! 7
,
, -
, colspan="2",
----
''Legend''
A pnictogen ( or ; from "to choke" and -gen, "generator") is any ...
ides (notable for its high melting point), , and , have various applications. Other binary compounds of zinc include zinc peroxide , zinc hydride , and zinc carbide .
Salts
Zinc nitrate (used asoxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
), zinc chlorate , zinc sulfate (known as "white vitriol
Vitriol is the general chemical name encompassing a class of chemical compounds comprising sulfates of certain metalsoriginally, iron or copper. Those mineral substances were distinguished by their color, such as green vitriol for hydrated iron(I ...
"), zinc phosphate (used as primer pigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored sub ...
), zinc molybdate (used as white pigment), zinc chromate (one of the few colored zinc compounds), zinc arsenite Zn(AsO2)2 (colorless powder) and zinc arsenate octahydrate (white powder, also referred to akoettigite
are a few examples of other common inorganic compounds of zinc. The latter two compounds are both used in insecticides and wood preservatives. One of the simplest examples of an
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
of zinc is zinc acetate , which has several medicinal applications. Zinc salts are usually fully dissociated in aqueous solution. Exceptions occur when the anion can form a complex, such as in the case of zinc sulfate
Zinc sulfate is an inorganic compound with the formula ZnSO4. It forms hydrates ZnSO4·''n''H2O, where ''n'' can range from 0 to 7. All are colorless solids. The most common form includes water of crystallization as the heptahydrate, with the che ...
, where the complex n(H2O)n(SO4may be formed, ( log K = ca. 2.5).
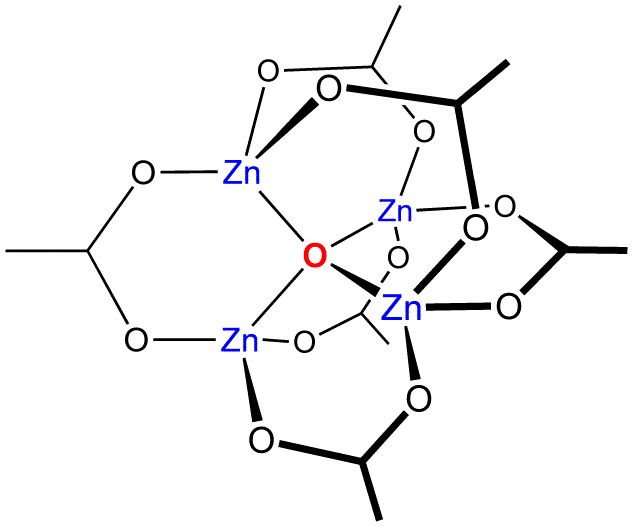
Complexes
 ]
The most common structure of zinc complexes is tetrahedral. Nevertheless, octahedral complexes comparable to those of the earlier transition metals are not rare. Zn2+ is a stability constants of complexes#Classification of metal ions, class A acceptor in the classification of Ahrland, Chatt and Davies, and so forms stronger complexes with the first-row donor atoms oxygen or nitrogen than with second-row sulfur or phosphorus. In terms of
]
The most common structure of zinc complexes is tetrahedral. Nevertheless, octahedral complexes comparable to those of the earlier transition metals are not rare. Zn2+ is a stability constants of complexes#Classification of metal ions, class A acceptor in the classification of Ahrland, Chatt and Davies, and so forms stronger complexes with the first-row donor atoms oxygen or nitrogen than with second-row sulfur or phosphorus. In terms of HSAB
HSAB is an acronym for "hard and soft (Lewis) acids and bases". HSAB is widely used in chemistry for explaining the stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chemical ...
theory Zn2+ is a hard acid.
In aqueous solution an octahedral complex, n(H2O)6sup>2+ is the predominant species. Aqueous solutions of zinc salts are mildly acidic because the aqua-ion is subject to hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
with a p''K''a of around 9, depending on conditions.
: n(H2O)6sup>2+ n(H2O)5(OH)sup>+ + H+
Hydrolysis explains why basic salts such as basic zinc acetate
Zinc acetate is a Salt (chemistry), salt with the chemical formula, formula Zn(CH3CO2)2, which commonly occurs as the Hydrate, dihydrate Zn(CH3CO2)2·2H2O. Both the hydrate and the anhydrous forms are colorless solids that are used as dietary supp ...
and basic zinc carbonate, Zn3(OH)4(CO3)•H2O are easy to obtain. The reason for the hydrolysis is the high electrical charge density on the zinc ion, which pulls electrons away from an OH bond of a coordinated water molecule and releases a hydrogen ion. The polarizing effect of Zn2+ is part of the reason why zinc is found in enzymes such as carbonic anhydrase
The carbonic anhydrases (or carbonate dehydratases) () form a family of enzymes that catalyst, catalyze the interconversion between carbon dioxide and water and the Dissociation (chemistry), dissociated ions of carbonic acid (i.e. bicarbonate a ...
.
No fluoro complexes are known, but complexes with the other halides and with pseudohalides, nX3sup>− and nX4sup>2− can be prepared. The case of the thiocyanate
Thiocyanates are salts containing the thiocyanate anion (also known as rhodanide or rhodanate). is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) t ...
complex illustrates the class A character of the zinc ion as it is the N-bonded isomer, n(NCS)4sup>2−in contrast to d(SCN)4sup>2− which is S-bonded. Being a class-A acceptor does not preclude the formation of complexes with sulfur donors, as is shown by zinc dithiophosphate
Zinc dialkyldithiophosphates (often referred to as ZDDP) are a family of coordination compounds developed in the 1940s that feature zinc bound to the anion of a dialkyldithiophosphoric salt (e.g., ammonium diethyl dithiophosphate). These uncharge ...
and the zinc finger complex (below).
The zinc acetylacetonate complex, Zn(acac)2 is interesting. As the ligand is bidentate a tetrahedral structure might be expected. However, the compound is in fact a trimer, Zn3(acac)6 in which each Zn ion is coordinated by five oxygen atoms in a distorted trigonal bipyramidal structure. Other 5-coordinate structures can be designed by choosing ligands which have specific stereochemical requirements. For example, terpyridine
Terpyridine (2,2';6',2"-terpyridine, often abbreviated to Terpy or Tpy) is a heterocyclic compound derived from pyridine. It is a white solid that is soluble in most organic solvents. The compound is mainly used as a ligand in coordination chemist ...
, which is a tridentate ligand forms the complex n(terpy)Cl2 Another example would involve a tripodal ligand
Tripodal ligands are tridentate, tri- and tetradentate ligands. They are popular in research in the areas of coordination chemistry and homogeneous catalysis. Because the ligands are polydentate, they do not readily dissociate from the metal centre ...
such as Tris(2-aminoethyl)amine. Square pyramidal 5-coordinate Zinc is found in Tetra(4-pyridyl)porphinatomonopyridinezinc(II) Solution studies of other 5-coordinate Zinc porphyrin
Porphyrins ( ) are heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (). In vertebrates, an essential member of the porphyrin group is heme, w ...
s have been reported. The compound zinc cyanide, Zn(CN)2, is not 2-coordinate. It adopts a polymeric structure consisting of tetrahedral zinc centres linked by bridging cyanide ligands. The cyanide group shows head to tail disorder with any zinc atom having between 1 and 4 carbon atom neighbours and the remaining being nitrogen atoms. These two examples illustrate the difficulty of sometimes relating structure to stoichiometry.
A coordination number of 2 occurs in zinc amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
Zn(NR1R2)2 (R1=CMe3, R2=SiMe3); the ligand is so bulky that there is not enough space for more than two of them.
Bio-complexes

 A very large number of metallo-enzymes contain zinc(II). Also many
A very large number of metallo-enzymes contain zinc(II). Also many protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s contain zinc for structural reasons. The zinc ion is invariably 4-coordinate with at least three ligands that are amino-acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
side-chains. The imidazole
Imidazole (ImH) is an organic compound with the formula . It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole.
Many natural products, ...
nitrogen of a histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an Amine, α-amino group (which is in the protonated –NH3+ form under Physiological condition, biological conditions), a carboxylic ...
side-chain is a common ligand. The following are typical examples of the two kinds of zinc-protein complexes.
In the active site of resting carbonic anhydrase
The carbonic anhydrases (or carbonate dehydratases) () form a family of enzymes that catalyst, catalyze the interconversion between carbon dioxide and water and the Dissociation (chemistry), dissociated ions of carbonic acid (i.e. bicarbonate a ...
a zinc ion is coordinated by three histidine residues. The fourth position is occupied by a water molecule, which is strongly polarized as in hydrolysis (see above). When carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
enters the active site, it subject to nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
attack by the oxygen atom which carries a partial negative charge, or indeed a full negative charge if the water molecule is dissociated. The CO2 is rapidly converted into a bicarbonate ion.
: -hys)3Zn(H2O)sup>2+ + CO2 → -hys)3Znsup>2+ + HCO3− + H+
Some peptidase
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do ...
s, such as glutamate carboxypeptidase II are thought to act in a similar way, with the zinc ion promoting the formation of a nucleophilic reagent.
The zinc finger
A zinc finger is a small protein structural motif that is characterized by the coordination of one or more zinc ions (Zn2+) which stabilizes the fold. The term ''zinc finger'' was originally coined to describe the finger-like appearance of a ...
motif is a rigid substructure in a protein which facilitates the binding of the protein to another molecule such as DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
. In this case all four coordination positions are occupied by the histidine and cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
residues. The tetrahedral geometry around the zinc ion constrains an α helix
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix).
The alpha helix is the most common structural arrangement in the secondary structure of proteins. It is also the most extreme type of l ...
fragment and an antiparallel β sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a gene ...
fragment to a particular orientation with respect to each other.
The magnesium ion, which has a higher concentration in biological fluids, cannot perform these functions because its complexes are much weaker than those of zinc.
Organometallic compounds

Organozinc compound
Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.The Chemistry of Organozinc Compounds' (Patai S ...
s contain zinc—carbon covalent bonds. Diethylzinc
Diethylzinc, or DEZ, is an organozinc compound with the chemical formula . It is highly pyrophoric and reactive, consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry. It is avail ...
() was first reported in 1848. It was made by reaction of zinc and ethyl iodide
Ethyl iodide (also iodoethane) is a transparency and translucency, colorless flammable chemical compound. It has the chemical formula C2H5I and is prepared by heating ethanol with iodine and phosphorus.''Merck Index of Chemicals and Drugs'', 9th e ...
and is the first compound known to contain a metal—carbon sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
. For a long time it was a mystery why copper(II) did not form an analogous compound. It was not until the 1980s that the reason was found: the zinc compound does not undergo the beta-hydride elimination reaction whereas the compound of the transition metal copper does so. Alkyl and aryl zinc compounds are contain the linear C—Zn—C motif. Because the zinc centre is coordinatively unsaturated, the compounds are powerful electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
s. In fact the low-molecular weight compounds will ignite spontaneously on contact with air and are immediately destroyed by reaction with water molecules. The use of zinc alkyls has been largely superseded by the use of the more easily handled Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
s. This demonstrates yet another connection between the chemistries of zinc and magnesium.
Zinc cyanide, , is used as a catalyst in some organic reactions.
Organometallic compounds of zinc(I) contain M—M bonds. Decamethyldizincocene
Decamethyldizincocene is an organozinc compound with the formula n2(η5–C5Me5)2 It is the first and an unusual example of a compound with a Zn-Zn bond. Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the prese ...
is now known.
See also
*Cadmium zinc telluride Cadmium zinc telluride, (CdZnTe) or CZT, is a compound of cadmium, zinc and tellurium or, more strictly speaking, an alloy of cadmium telluride and zinc telluride. A direct bandgap semiconductor, it is used in a variety of applications, including S ...
*Mercury cadmium telluride
Hg1−''x''Cd''x''Te or mercury cadmium telluride (also cadmium mercury telluride, MCT, MerCad Telluride, MerCadTel, MerCaT or CMT) is a chemical compound of cadmium telluride (CdTe) and mercury telluride (HgTe) with a tunable bandgap spanning th ...
*Zinc gluconate
Zinc gluconate is the zinc salt of gluconic acid. It is an ionic compound consisting of two anions of gluconate for each zinc(II) cation. Zinc gluconate is a popular form for the delivery of zinc as a dietary supplement providing 14.35% element ...
*Zinc pyrithione
Zinc pyrithione (or pyrithione zinc) is a coordination complex of zinc. It has fungistatic (inhibiting the division of fungal cells) and bacteriostatic (inhibiting bacterial cell division) properties and is used in the treatment of seborrhoeic ...
*Zinc ricinoleate
Zinc ricinoleate is the zinc salt of ricinoleic acid, a major fatty acid found in castor oil. It is used in many deodorant
A deodorant is a substance applied to the body to prevent or mask body odor caused by bacterial breakdown of perspiratio ...
* Zinc stearate
*Zinc pest
Zinc pest (from German ''Zinkpest'' "zinc plague"), also known as zinc rot, mazak rot and zamak rot, is a destructive, intercrystalline corrosion process of zinc alloys containing lead impurities. Prepared under the direction of the ASM Interna ...
References
{{Authority control Z simple:Zinc#Chemical compounds