 |
Tripodal Ligand
Tripodal ligands are tridentate, tri- and tetradentate ligands. They are popular in research in the areas of coordination chemistry and homogeneous catalysis. Because the ligands are polydentate, they do not readily dissociate from the metal centre. Many tripodal ligands have C3 symmetry group, symmetry. Coordination chemistry In their coordination complexes with an octahedral molecular geometry the tridentate tripod ligands occupy one face, leading to a fixed Coordination complexes#Cis–trans isomerism and facial–meridional isomerism, facial (or ''fac'') geometry. The tetradentate tripodal ligands occupy four contiguous sites, leaving two ''cis'' positions available on the octahedral metal center. When bound to four- and five-coordinate metal centres, these ligands impose C3 symmetry, which can lead to uncommon ligand field splitting patterns. Tripodal ligands are often able to coordinately saturate metal ions with lower coordination numbers. One tripodal ligand of commercial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Tris(2-aminoethyl)amine
Tris(2-aminoethyl)amine is the organic compound with the chemical formula, formula N(CH2CH2NH2)3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups. Tris(2-aminoethyl)amine is commonly abbreviated as tren or TREN. It is used a crosslinking agent in the synthesis of polyimine networks and a tripodal ligand in coordination chemistry. Supramolecular and polymer derivatives Tris(2-aminoethyl)amine has been used to prepare molecular capsules and related supramolecular structures. Metal complexes Tren is a C3-symmetric, tetradentate chelating ligand that forms stable complexes with transition metals, especially those in the 2+ and 3+ oxidation states. Tren complexes exist with relatively few isomers, reflecting the constrained connectivity of this tetramine. Thus, only a single achiral stereoisomer exists for [Co(tren)X2]+, where X is halide or pseudohalide. In contrast, for [Co(trien)X2]+ five di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
 |
Cis,cis-1,3,5-Triaminocyclohexane
''cis'',''cis''-1,3,5-Triaminocyclohexane is an organic compound with the formula (CH2CHNH2)3. It is a triamine. Of the many isomers possible for triaminocyclohexane, the ''cis'',''cis''-1,3,5-derivative has attracted attention because it is a common tripodal ligand, abbreviated as tach. It is a colorless oil. It is a popular tridentate ligand in coordination chemistry. It is prepared from the triscarbamate In organic chemistry, a carbamate is a category of organic compounds with the general Chemical formula, formula and Chemical structure, structure , which are formally Derivative (chemistry), derived from carbamic acid (). The term includes orga ... of cyclohexane. The latter is generated via the Curtius rearrangement starting from cyclohexanetricarboxylic acid. Related ligands * Tris(aminomethyl)ethane, another tripodal triamine (CH3C(CH2NH2]3) References {{DEFAULTSORT:Triaminocyclohexane, cis,cis-1,3,5- Tripodal ligands Polyamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
1,1,1-Tris(aminomethyl)ethane
1,1,1-Tris(aminomethyl)ethane (TAME) is an organic compound with the formula CHC(CHNH). It is a colorless liquid. It is classified as a polyamine tripodal ligand, i.e., capable of binding to metal ions through three sites and hence is a tridentate chelating ligand, occupying a face of the coordination polyhedron. Preparation TAME is synthesized by the Pd/C-catalyzed hydrogenation of 1,1,1-tris(azidomethyl)ethane. Although azides are potentially explosive, they are excellent and practical source of primary amines. The required tris(azidomethyl)ethane is obtained from the tri tosylate by salt metathesis using sodium azide. These two steps are: :3 NaN + CHC(CHOTs) → CHC(CHN) + 3 NaOTs :3 H + CHC(CHN) → CHC(CHNH) + 3 N Complexes of TAME The tripodal TAME ligand In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Kläui Ligand
The Kläui ligand is the anion −. The ligand, popularized by Wolfgang Kläui, binds metals and metalloids via a facial O3 donor set. Related tridentate and tripodal anionic ligands include trispyrazolylborates. : 160px, General structure of a metal center (MLn) coordinated to a κ3-Kläui ligand. The ligand is derived from the cationic complex of trimethylphosphite 2+ via an Arbuzov reaction. Using other phosphites and other cyclopentadienyl Cyclopentadienyl can refer to * Cyclopentadienyl anion, or cyclopentadienide, ** Cyclopentadienyl ligand * Cyclopentadienyl radical, • * Cyclopentadienyl cation, See also * Pentadienyl {{Chemistry index ... ligands, a large variety of derivatives are possible. The parent acid H is highly soluble in water (270 g/100 mL). Its p''K''a is about 2. Many complexes have been described, including bis( chelate) complexes of the type {M (C5H5)Co[(CH3O)2POsub>3}2]n+ (M = Co(II), Mn(II), Bi(III), etc.).Wolfgang Kläui, Heiko Otto, Werner ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
 |
Trispyrazolylborate
The trispyrazolylborate ligand, abbreviated Tp−, is an anionic Denticity, tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion [HB(C3N2H3)3]−. However, the term can also be used to refer to derivatives having substituents on the pyrazolyl rings. This class of compounds belongs to the family of ligands called scorpionate ligands. Tp ligands As suggested by the resonance structures, the nitrogen centers that are not bonded to boron are basic. These centers bind to three adjacent sites of a metal such that the simple adducts have C3v symmetry group, symmetry. The facial bonding mode is reminiscent of cyclopentadienyl ligands, although the ligand field stabilization energy of Tp− is weaker as indicated by the fact that Fe(Tp)2 is a spin crossover, spin-crossover complex whereas ferrocene is low-spin. The Tp ligands are usually prepared from the reaction of pyrazole with potassium borohydride: :KBH4 + 3 C3H3N2H → K[HB(C3N2H3)3] + 3H2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Bioinorganic Chemistry
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those that are non-essential, in medicine and toxicology. Many biological processes such as respiration depend upon molecules that fall within the realm of inorganic chemistry. The discipline also includes the study of inorganic models or mimics that imitate the behaviour of metalloproteins. As a mix of biochemistry and inorganic chemistry, bioinorganic chemistry is important in elucidating the implications of electron-transfer proteins, substrate bindings and activation, atom and group transfer chemistry as well as metal properties in biological chemistry. The successful development of truly interdisciplinary work is necessary to advance bioinorganic chemistry. Composition of living organisms About 99% of mammals' mass are the elements carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Tris(2-pyridylmethyl)amine
Tris(2-pyridylmethyl)amine (abbreviated TPMA or TPA) is an organic compound with the formula (C5H4NCH2)3N. It is a tertiary amine with three picolyl substituents. It is a white solid that is soluble in polar organic solvents. It is a ligand in coordination chemistry. The ligand is prepared by the alkylation of 2-picolylamine by picolyl chloride:{{cite book , author1=James W. Canary , author2=Yihan Wang , author3=Richard Roy, Jr. , title=Inorganic Syntheses , chapter=Tris[(2-Pyridyl)Methyl] Amine (TPA) and (+)-Bis[(2-Pyridyl)methyl]-1-(2-Pyridyl)-Ethylamine (α-Metpa) , journal = Inorg. Synth. , year = 1998 , volume = 32 , pages = 70–75 , doi = 10.1002/9780470132630.ch11, isbn=978-0-470-13263-0 :2 C5H4NCH2Cl + C5H4NCH2NH2 → (C5H4NCH2)3N + 2 HCl TPMA is a tripodal ligand, often used to simulate the coordination environment within some proteins. It is also used as a copper ligand in ATRP. Related ligands * dipicolylamine, an intermediate in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
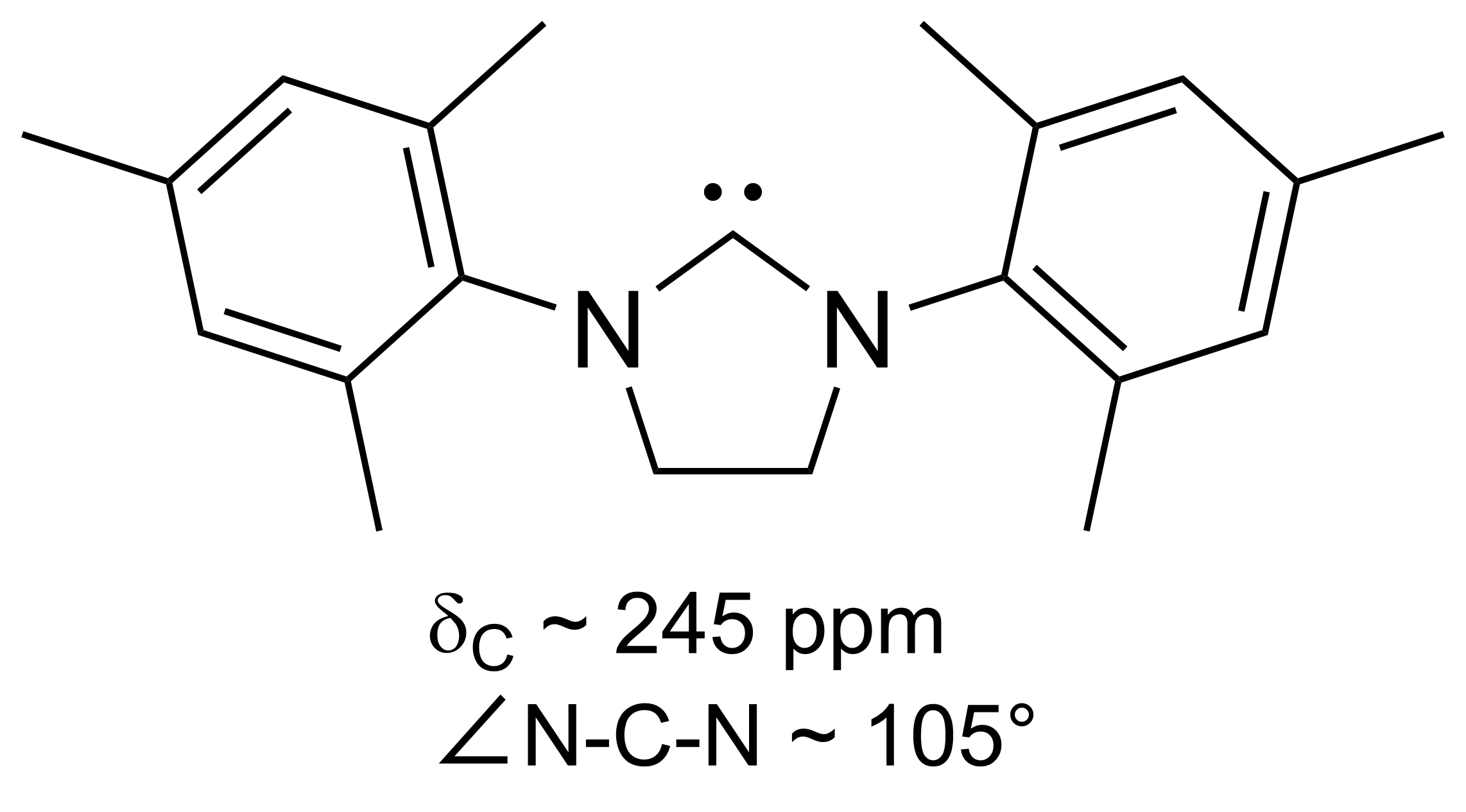 |
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), in which nitrogen atoms flank the formal carbene. Modern theoretical analysis suggests that the term "persistent carbene" is in fact a misnomer. Persistent carbenes do not in fact have a carbene electronic structure in their ground state, but instead an ylide stabilized by Aromaticity, aromatic resonance or steric shielding. Excitation to a carbene structure then accounts for the carbene-like dimerization that some persistent carbenes undergo over the course of days. Persistent carbenes in general, and Arduengo carbenes in particular, are popular ligands in organometallic chemistry. Histor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
1,1,1-Tris(diphenylphosphinomethyl)ethane
1,1,1-Tris(diphenylphosphinomethyl)ethane, also called Triphos, is an organophosphorus compound with the formula CH3C H2PPh2sub>3. An air-sensitive white solid, it is a tripodal ligand ("three-legged") of idealized C3v symmetry. It was originally prepared by the reaction of sodium diphenylphosphide and CH3C(CH2Cl)3: :3 Ph2PNa + CH3C(CH2Cl)3 → CH3C H2PPh2sub>3 + 3 NaCl It forms complexes with many transition metals, usually as a tripodal ligand. Such complexes are used to analyze mechanistic aspects of homogeneous catalysts. For example, rhodium forms complexes with CH3C H2PPh2sub>3 like triphos)RhCl(C2H4) triphos)RhH(C2H4) and triphos)Rh(C2H5)(C2H4) provide model intermediates in the catalytic cycle for hydrogenation of alkenes. Triphos sometimes behaves as a bidentate ligand. Illustrative cases include ''fac''- n(CO)3Br(η2-triphos)and (CO)4(η2-triphos) where M is Cr, Mo, or W. Triphos serves as a tridentate-bridging ligand in an icosahedral In geometry, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Nitrilotriacetic Acid
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid. Its conjugate base nitrilotriacetate is used as a chelating agent for Ca2+, Co2+, Cu2+, and Fe3+. Production and use Nitrilotriacetic acid is commercially available as the free acid and as the sodium salt. It is produced from ammonia, formaldehyde, and sodium cyanide or hydrogen cyanide. NTA is also cogenerated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia coproduct. Older routes to NTA included alkylation of ammonia with chloroacetic acid and oxidation of triethanolamine. Coordination chemistry and applications The conjugate base of NTA is a tripodal tetradentate trianionic ligand, forming coordination compounds with a variety of metal ions. Like EDTA, its sodium salt is used for water softening to remove Ca2+. For this purpose, NTA is a replacement for triphosphate, which once was widely used in detergents, and cleansers, but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |