|
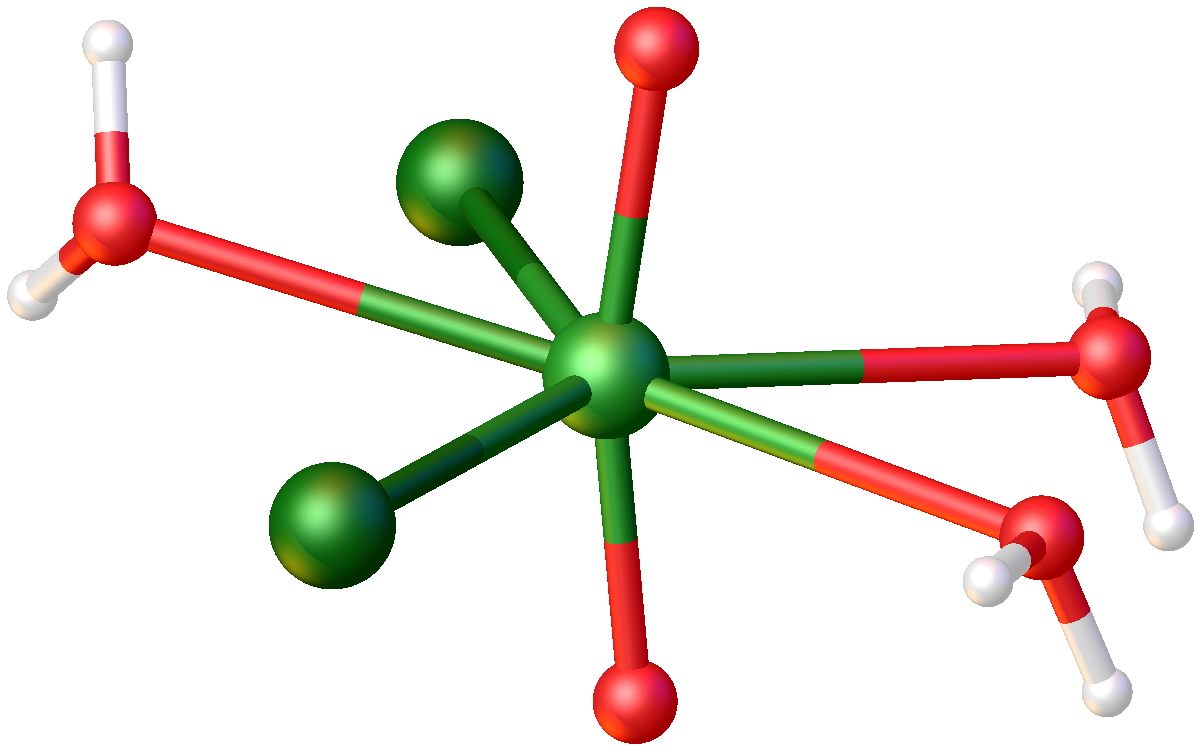
Uranyl Sulfate
Uranyl sulfate describes a family of inorganic compounds with the formula UO2SO4(H2O)n. These salts consist of sulfate, the uranyl ion, and water. They are lemon-yellow solids. Uranyl sulfates are intermediates in some extraction methods used for uranium ores. These compounds can also take the form of an anhydrous salt. Structure The structure of UO2(SO4)(H2O)3.5 is illustrative of the uranyl sulfates. The ''trans''-UO22+ centers are encased in a pentagonal bipyramidal coordination sphere. In the pentagonal plane are five oxygen ligands derived from sulfate and aquo ligands. The compound is a coordination polymer. Uses Aside from the large scale use in mining, uranyl sulfate finds some use as a negative stain in microscopy and Radioactive tracer, tracer in biology. The Aqueous Homogeneous Reactor experiment, constructed in 1951, circulated a fuel composed of 565 grams of Uranium-235, U-235 enriched uranium, enriched to 14.7% in the form of uranyl sulfate. The acid process of mill ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranyl Chloride
Uranyl chloride is a chemical compound with the chemical formula . It consists of uranyl cations and chloride anions . It is fluorescent. Uranyl chloride also refers to inorganic compounds with the formula where ''n'' = 0, 1, 2, or 3. These are yellow salts. Synthesis and structures The hydrates are obtained by dissolving uranyl sulfate or uranyl acetate in hydrochloric acid followed by crystallization from concentrated solutions. Depending on the method of drying, one obtains the mono- or the trihydrate. The monohydrate is described as a yellow, sulfur-like powder. It is very hygroscopic. The trihydrate is greenish-yellow. Both hydrates are fluorescent solids that are highly soluble in water. The anhydrous material can be obtained by the reaction of oxygen with uranium tetrachloride: : In terms of structures, all three of these compounds feature the uranyl center (''trans''-) bound to five additional ligands, which can include (bridging) chloride, water, or another uranyl oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqueous Homogeneous Reactor
Aqueous homogeneous reactors (AHR) is a two (2) chamber reactor consisting of an interior reactor chamber and an outside cooling and moderating jacket chamber. They are a type of nuclear reactor in which soluble nuclear salts (usually uranium sulfate or uranium nitrate) are dissolved in water. The fuel is mixed with heavy or light water which partially moderates and cools the reactor. The outside layer of the reactor has more water which also partially cools and acts as a moderator. The water can be either heavy water or ordinary (light) water, which slows neutrons and helps facilitate a stable reaction, both of which need to be very pure. Their self-controlling features and ability to handle very large increases in reactivity make them unique among reactors, and possibly safest. At Santa Susana, California, Atomics International performed a series of tests titled The Kinetic Energy Experiments. In the late 1940s, control rods were loaded on springs and then flung out of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranyl Compounds
The uranyl ion with the chemical formula has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen, with uranium in the oxidation state +6. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing. Structure and bonding The uranyl ion is linear and symmetrical, specifically belonging to the D∞h point group, with both U–O bond lengths of about 180 pm. The bond lengths are indicative of the presence of multiple bonding between the uranium and oxygen atoms. Since uranium(VI) has the electronic configuration of the preceding noble gas, radon, the electrons used in forming the U–O bonds are supplied by the oxygen atoms. The electrons are donated into empty atomic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactivity
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henri Becquerel
Antoine Henri Becquerel ( ; ; 15 December 1852 – 25 August 1908) was a French nuclear physicist who shared the 1903 Nobel Prize in Physics with Marie and Pierre Curie for his discovery of radioactivity. Biography Family and education Becquerel was born in Paris, France, into a wealthy family which produced four generations of notable physicists, including Becquerel's grandfather ( Antoine César Becquerel), father ( Edmond Becquerel), and son ( Jean Becquerel). Henri started off his education by attending the Lycée Louis-le-Grand school, a prep school in Paris. He studied engineering at the École polytechnique and the École des ponts et chaussées. Career Becquerel's earliest works centered on the subject of his doctoral thesis: the plane polarization of light, with the phenomenon of phosphorescence and absorption of light by crystals. Becquerel was awarded a Doctor of Science in 1888. Early in his career, Becquerel also studied the Earth's magnetic field. Becqu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogensulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry of the isolated anion is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yellowcake
Yellowcake (also called urania) is a type of powdered uranium concentrate obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C. Overview Originally, raw uranium ore was extracted by traditional mining, and this is still the case in many mines. It is first crushed to a fine powder by passing it through crushers and grinders to produce "pulped" ore. This is further processed with concentrated acid, alkaline, or peroxide solutions to leach out the uranium. However, nearl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaching (chemical Science)
Leaching is the process of a solute becoming detached or extracted from its carrier substance by way of a solvent. Leaching is a naturally occurring process which scientists have adapted for a variety of applications with a variety of methods. Specific extraction methods depend on the soluble characteristics relative to the sorbent material such as concentration, distribution, nature, and size. Leaching can occur naturally seen from plant substances (inorganic and organic), solute leaching in soil, and in the decomposition of organic materials. Leaching can also be applied affectedly to enhance water quality and contaminant removal, as well as for disposal of hazardous waste products such as fly ash, or rare earth elements (REEs). Understanding leaching characteristics is important in preventing or encouraging the leaching process and preparing for it in the case where it is inevitable. In an ideal leaching equilibrium stage, all the solute is dissolved by the solvent, leaving t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enriched Uranium
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U with 99.2732–99.2752% natural abundance), uranium-235 (235U, 0.7198–0.7210%), and uranium-234 (234U, 0.0049–0.0059%). 235U is the only nuclide existing in nature (in any appreciable amount) that is fissile with thermal neutrons. Enriched uranium is a critical component for both civil nuclear power generation and military nuclear weapons. Low-enriched uranium (20% 235U, typically >85%) is used for the cores of many nuclear weapons, as well as compact reactors for naval propulsion and research, as well as breeder reactors. There are about 2,000 tonnes of highly enriched uranium in the world. Enrichment methods were first developed on a large scale by the Manhattan Project. Its gaseous diffusion method was used in the 194 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nature as a primordial nuclide. Uranium-235 has a half-life of 703.8 million years. It was discovered in 1935 by Arthur Jeffrey Dempster. Its fission cross section for slow thermal neutrons is about Barn (unit), barns. For fast neutrons it is on the order of 1 barn. Most neutron absorptions induce fission, though a minority (about 15%) result in the formation of uranium-236. Fission properties The fission of one atom of uranium-235 releases () inside the reactor. That corresponds to 19.54 TJ/mole (unit), mol, or 83.14 TJ/kg. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biology
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, History of life, origin, evolution, and distribution of life. Central to biology are five fundamental themes: the cell (biology), cell as the basic unit of life, genes and heredity as the basis of inheritance, evolution as the driver of biological diversity, energy transformation for sustaining life processes, and the maintenance of internal stability (homeostasis). Biology examines life across multiple biological organisation, levels of organization, from molecules and cells to organisms, populations, and ecosystems. Subdisciplines include molecular biology, physiology, ecology, evolutionary biology, developmental biology, and systematics, among others. Each of these fields applies a range of methods to investigate biological phenomena, including scientific method, observation, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |