uranyl chloride on:
[Wikipedia]
[Google]
[Amazon]
Uranyl chloride is a chemical compound with the
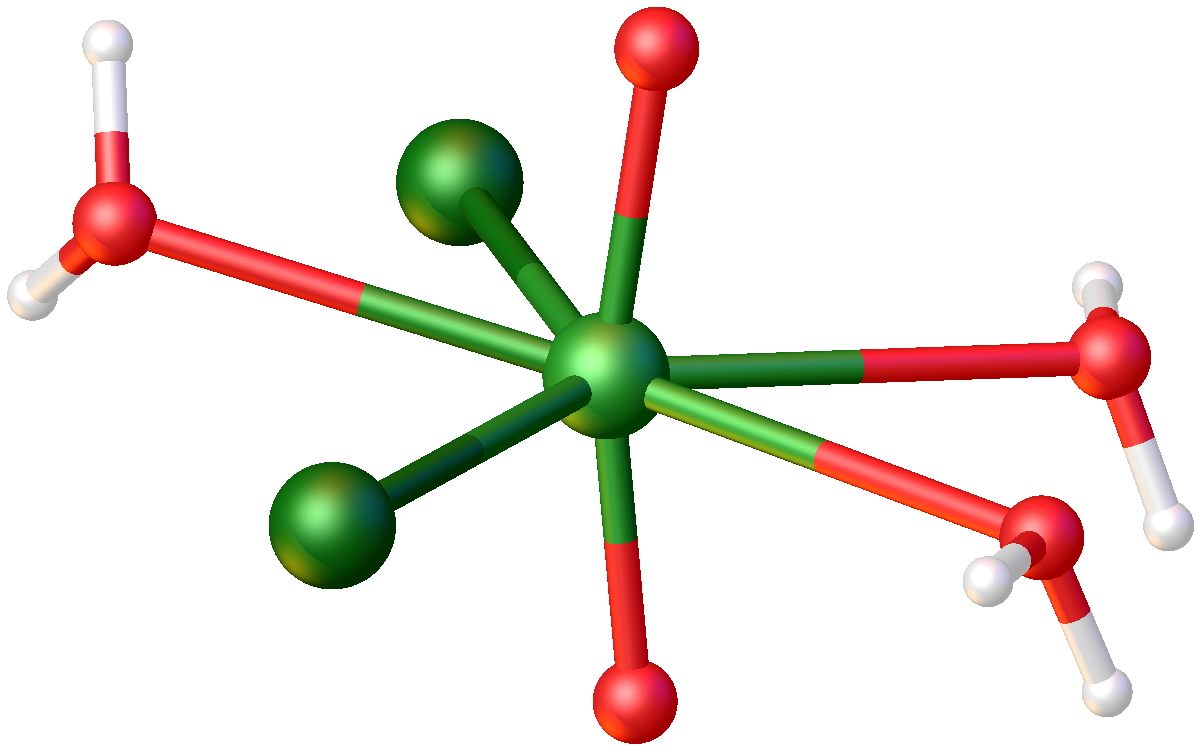 The hydrates are obtained by dissolving
The hydrates are obtained by dissolving
chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
. It consists of uranyl
The uranyl ion with the chemical formula has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen, with uranium in the oxidation state +6. Four or more ligands may be bound to the u ...
cations
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
and chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
. It is fluorescent
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with color ...
. Uranyl chloride also refers to inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
s with the formula where ''n'' = 0, 1, 2, or 3. These are yellow salts.
Synthesis and structures
 The hydrates are obtained by dissolving
The hydrates are obtained by dissolving uranyl sulfate
Uranyl sulfate describes a family of inorganic compounds with the formula UO2SO4(H2O)n. These salts consist of sulfate, the uranyl ion, and water. They are lemon-yellow solids. Uranyl sulfates are intermediates in some extraction methods used for ...
or uranyl acetate
Uranyl acetate is the acetate salt of uranium oxide, a toxic yellow-green powder useful in certain laboratory tests. Structurally, it is a coordination polymer with formula UO2(CH3CO2)2(H2O)·H2O.
Structure
left, 260px, Structure (from X-ray ...
in hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
followed by crystallization from concentrated solutions. Depending on the method of drying, one obtains the mono- or the trihydrate. The monohydrate is described as a yellow, sulfur-like powder. It is very hygroscopic. The trihydrate is greenish-yellow. Both hydrates are fluorescent solids that are highly soluble in water.
The anhydrous material can be obtained by the reaction of oxygen with uranium tetrachloride
Uranium tetrachloride is an inorganic compound, a salt of uranium and chlorine, with the formula UCl4. It is a hygroscopic olive-green solid. It was used in the electromagnetic isotope separation (EMIS) process of uranium enrichment. It is one ...
:
:
In terms of structures, all three of these compounds feature the uranyl
The uranyl ion with the chemical formula has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen, with uranium in the oxidation state +6. Four or more ligands may be bound to the u ...
center (''trans''-) bound to five additional ligands, which can include (bridging) chloride, water, or another uranyl oxygen.
Reactions
Theaquo ligand
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant Chemical species, species in aqueous solutions of many metal Salt (chemistry), salts, such as meta ...
s can be replaced by a variety of donors, e.g. THF. Uranyl chloride, and its two hydrates, ( and ) decompose in the presence of light. This photosensitivity over the years, from time to time, has attracted scientific interest and there have been various unsuccessful attempts to develop applications in photography using these compounds.
Industrial importance
The company Indian Rare Earths Limited (IREL) has developed a process to extract uranium from the Western and Eastern coastal dune sands ofIndia
India, officially the Republic of India, is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area; the List of countries by population (United Nations), most populous country since ...
. After pre-processing with high-intensity magnetic separators and fine grinding, the mineral sand
Sand is a granular material composed of finely divided mineral particles. Sand has various compositions but is usually defined by its grain size. Sand grains are smaller than gravel and coarser than silt. Sand can also refer to a textural ...
s (known as monazite), are digested with caustic soda at about and water. The hydroxide concentrate is further digested with concentrated hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
to solubilise all hydroxides to form a feed solution composed of chlorides of uranium, rare earth element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set o ...
s and thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
. The solution is subjected to liquid–liquid extraction
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubility, solubilities in two different Miscibility, immiscible liquids, usually wate ...
with dual solvent systems to produce uranyl chloride and thorium oxalate. The crude uranyl chloride solution is subsequently refined to nuclear grade ammonium diuranate by a purification process involving precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
and solvent extraction
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
in a nitrate media.
Safety
Uranyl chloride is highly toxic by ingestion and inhalation. Cumulative toxic effects are also probable, with the target organs being the liver and the kidneys. It is toxic to aquatic organisms, and may cause long-term catastrophic effects in the aquatic environment. As all uranium compounds, uranyl chloride is radioactive.References
* *External links
* {{DEFAULTSORT:Uranyl Chloride Uranyl compounds Chlorides Metal halides Oxychlorides