|
TPST2
Tyrosylprotein sulfotransferase is an enzyme that catalyzes tyrosine sulfation. Function Tyrosylprotein sulfotransferase is the enzyme that catalyzes the sulfation reaction of protein tyrosines, a post-translational modification of proteins. It utilizes 3'-Phosphoadenosine-5'-phosphosulfate (PAPS) as the sulfonate donor and binds proteins with target tyrosine residues to eventually form the tyrosine O-sulfate ester group and the desulfonated 3’-phosphoadenosine-5’-phosphate (PAP). TPST and tyrosine sulfation is involved in a large number of biological and physiological processes. Tyrosine sulfation has been found to be an important part of the inflammatory process, leukocyte movement and cytosis, viral cell entrance, and other cell-cell and protein-protein interactions. Selection for specific tyrosine residues requires a generally accessible tyrosine residue, and acidic residues within +5 or -5 residues of the target tyrosine. P-selectin glycoprotein ligand-1 (PSGL-1) h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfotransferase
In biochemistry, sulfotransferases (SULTs) are transferase enzymes that catalyze the transfer of a sulfo group () from a donor molecule to an acceptor alcohol () or amine (). The most common sulfo group donor is 3'-phosphoadenosine-5'-phosphosulfate (PAPS). In the case of alcohol as acceptor, the product is a sulfate (): :\ce \ + \ \ce \quad \xrightarrowtext\quad \ce \ + \ \ce whereas an amine leads to a sulfamate (): :\ce \ + \ \ce \quad \xrightarrowtext\quad \ce \ + \ \ce Both reactive groups for a sulfonation via sulfotransferases may be part of a protein, lipid, carbohydrate or steroid. Examples The following are examples of sulfotransferases: * carbohydrate sulfotransferase: CHST1, CHST2, CHST3, CHST4, CHST5, CHST6, CHST7, CHST8, CHST9, CHST10, CHST11, CHST12, CHST13, CHST14 * galactose-3-O-sulfotransferase: GAL3ST1, GAL3ST2, GAL3ST3, GAL3ST4 * heparan sulfate 2-O-sulfotransferase: HS2ST1 * heparan sulfate 3-O-sulfotransferase: HS3ST1, HS3S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine Sulfation
In biochemistry, tyrosine sulfation is a posttranslational modification where a sulfate group () is added to a tyrosine residue of a protein molecule. Secreted proteins and extracellular parts of membrane proteins that pass through the Golgi apparatus may be sulfated. Sulfation was first discovered by Bettelheim in bovine fibrinopeptide B in 1954 and later found to be present in animals and plants but not in prokaryotes or in yeast. Function Sulfation plays a role in strengthening protein-protein interactions. Types of human proteins known to undergo tyrosine sulfation include adhesion molecules, G-protein-coupled receptors, coagulation factors, serine protease inhibitors, extracellular matrix proteins, and hormones. Tyrosine O-sulfate is a stable molecule and is excreted in urine in animals. No enzymatic mechanism of tyrosine sulfate desulfation is known to exist. By knock-out of TPST genes in mice, it may be observed that tyrosine sulfation has effects on the growth of the mice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TPST2
Tyrosylprotein sulfotransferase is an enzyme that catalyzes tyrosine sulfation. Function Tyrosylprotein sulfotransferase is the enzyme that catalyzes the sulfation reaction of protein tyrosines, a post-translational modification of proteins. It utilizes 3'-Phosphoadenosine-5'-phosphosulfate (PAPS) as the sulfonate donor and binds proteins with target tyrosine residues to eventually form the tyrosine O-sulfate ester group and the desulfonated 3’-phosphoadenosine-5’-phosphate (PAP). TPST and tyrosine sulfation is involved in a large number of biological and physiological processes. Tyrosine sulfation has been found to be an important part of the inflammatory process, leukocyte movement and cytosis, viral cell entrance, and other cell-cell and protein-protein interactions. Selection for specific tyrosine residues requires a generally accessible tyrosine residue, and acidic residues within +5 or -5 residues of the target tyrosine. P-selectin glycoprotein ligand-1 (PSGL-1) h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Follicle-stimulating Hormone Receptor
The follicle-stimulating hormone receptor or FSH receptor (FSHR) is a transmembrane receptor that interacts with the follicle-stimulating hormone (FSH) and represents a G protein-coupled receptor (GPCR). Its activation is necessary for the hormonal functioning of FSH. FSHRs are found in the ovary, testis, and uterus. FSHR gene The gene for the FSHR is found on chromosome 2 p21 in humans. The gene sequence of the FSHR consists of about 2,080 nucleotides. Receptor structure The FSHR consists of 695 amino acids and has a molecular mass of about 76 kDa. Like other GPCRs, the FSH-receptor possesses seven membrane-spanning domains or transmembrane helices. * The ''extracellular domain'' of the receptor contains 11 leucine-rich repeats and is glycosylated. It has two subdomains, a hormone-binding subdomain followed by a signal-specificity subdomain. The hormone-binding subdomain is responsible for the high-affinity hormone binding, and the signal-specificity subdomain, con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SN2 Reaction
The bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while the leaving group detaches from the reaction center in a concerted (i.e. simultaneous) fashion. The name SN2 refers to the Hughes-Ingold symbol of the mechanism: "SN" indicates that the reaction is a nucleophilic substitution, and "2" that it proceeds via a bimolecular mechanism, which means both the reacting species are involved in the rate-determining step. What distinguishes SN2 from the other major type of nucleophilic substitution, the SN1 reaction, is that the displacement of the leaving group, which is the rate-determining step, is separate from the nucleophilic attack in SN1. The SN2 reaction can be considered as an organic-chemistry analogue of the associative substitution from the field of inorganic chemistry. Reaction mech ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ping-pong Mechanism
Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or a modifier (inhibitor or activator) might affect the rate. An enzyme (E) is a protein molecule that serves as a biological catalyst to facilitate and accelerate a chemical reaction in the body. It does this through binding of another molecule, its substrate (S), which the enzyme acts upon to form the desired product. The substrate binds to the active site of the enzyme to produce an enzyme-substrate complex ES, and is transformed into an enzyme-product complex EP and from there to product P, via a transition state ES*. The series of steps is known as the mechanism: : E + S ⇄ ES ⇄ ES* ⇄ EP ⇄ E + P Thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionization, ionized, for example by bombarding it with a Electron ionization, beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosophila Melanogaster
''Drosophila melanogaster'' is a species of fly (an insect of the Order (biology), order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly", "pomace fly", or "banana fly". In the wild, ''D. melanogaster'' are attracted to rotting fruit and fermenting beverages, and are often found in orchards, kitchens and pubs. Starting with Charles W. Woodworth's 1901 proposal of the use of this species as a model organism, ''D. melanogaster'' continues to be widely used for biological research in genetics, physiology, microbial pathogenesis, and Life history theory, life history evolution. ''D. melanogaster'' was the first animal to be Fruit flies in space, launched into space in 1947. As of 2017, six Nobel Prizes have been awarded to drosophilists for their work using the insect. ''Drosophila melanogaster'' is typically used in research owing to its rapid life cycle, relatively simple genetics with on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from alternative splicings, variable promoter usage, or other post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. (For that, see Proteoforms.) Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments ( exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein. The discovery of isoforms could explain the discrepancy between the small number of protein coding regions of genes revealed by the human genome project and the large diversity of proteins seen in an organism: different proteins e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
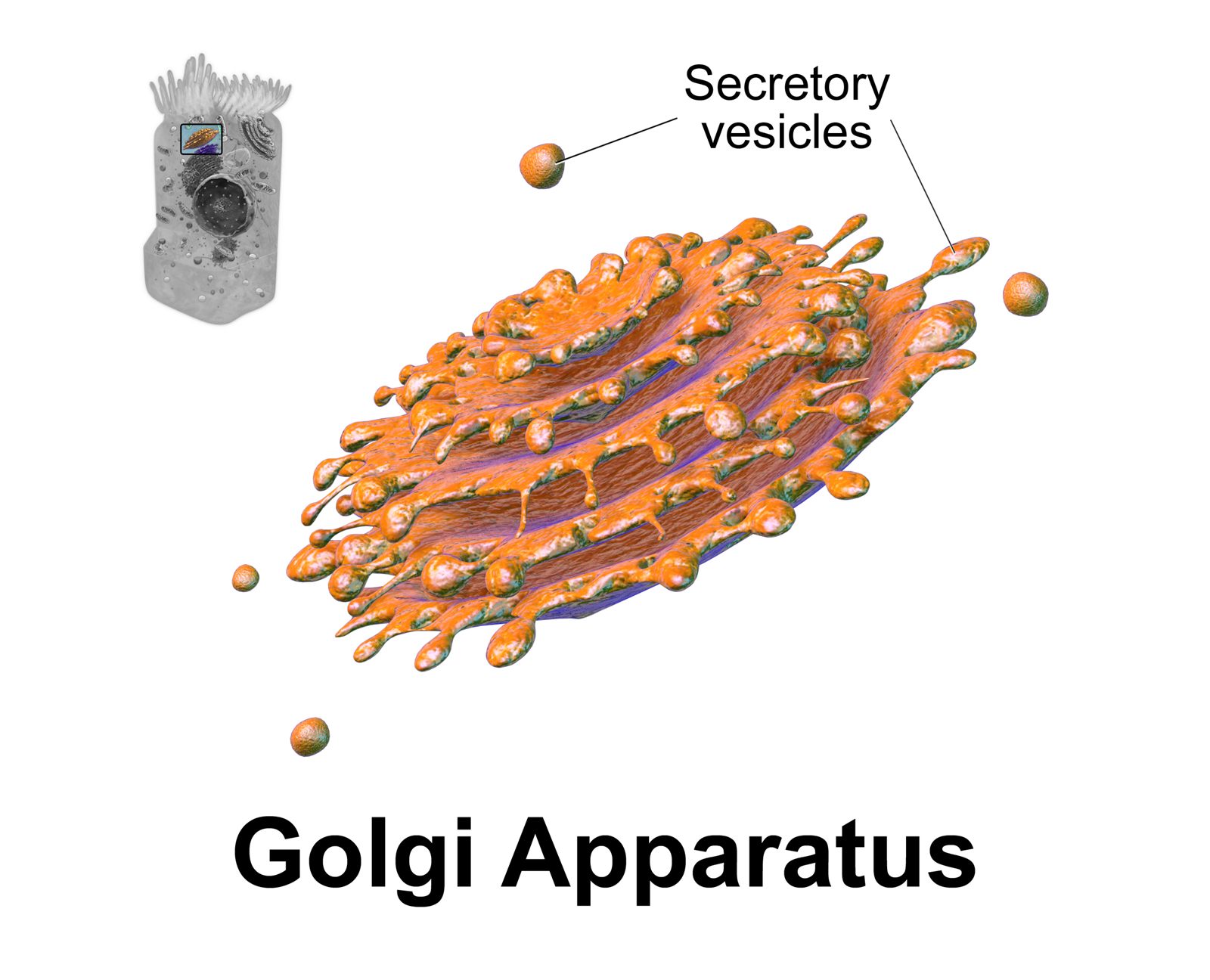
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic Cell (biology), cells. Part of the endomembrane system in the cytoplasm, it protein targeting, packages proteins into membrane-bound Vesicle (biology and chemistry), vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and Endocytosis, endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. The Golgi apparatus was identified in 1898 by the Italian biologist and pathologist Camillo Golgi. The organelle was later named after him in the 1910s. Discovery Because of its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Protein
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them ( beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-pass membrane proteins, or as multipass membrane proteins. Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |