|
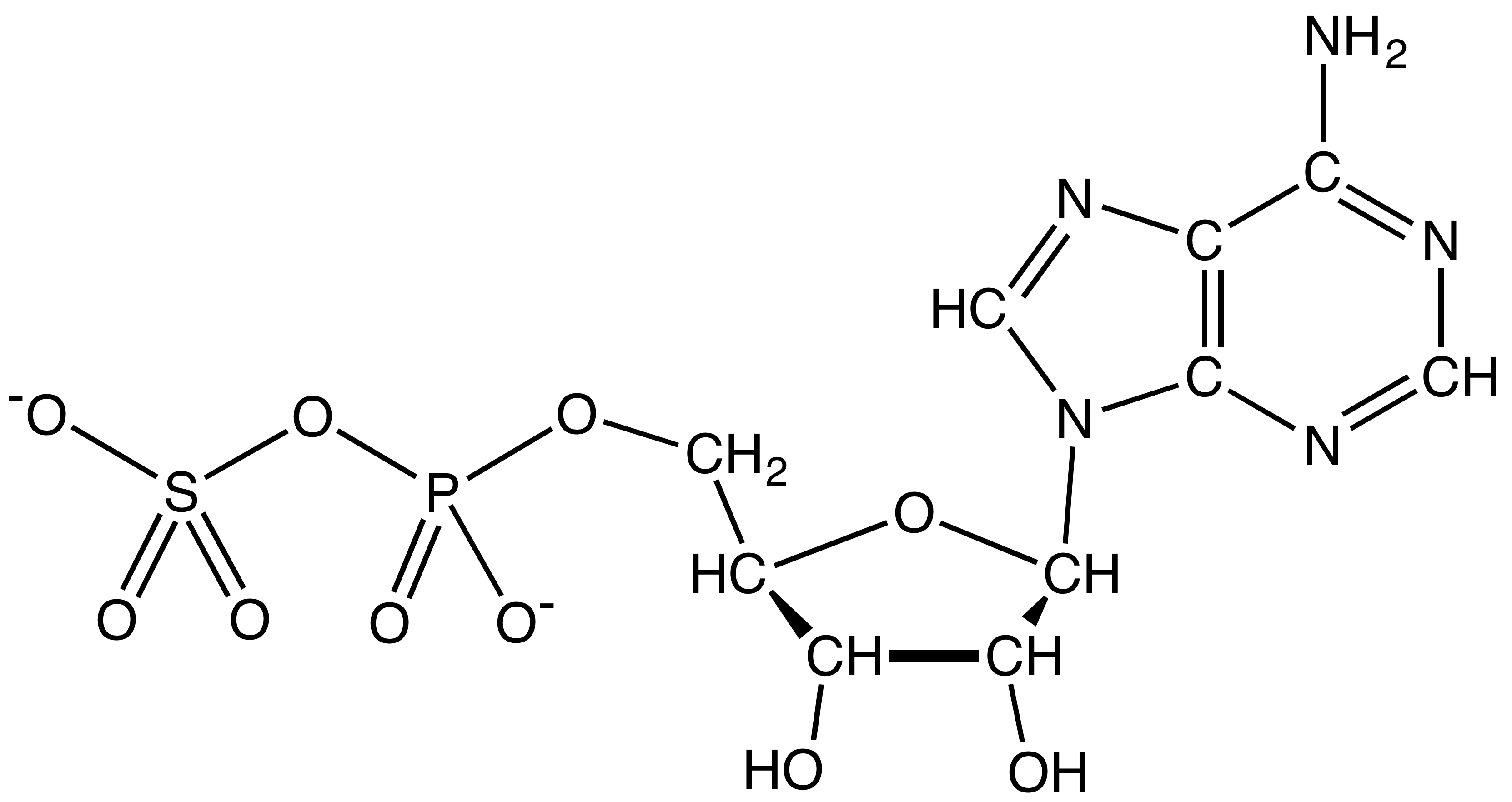
Tyrosine Sulfation
In biochemistry, tyrosine sulfation is a posttranslational modification where a sulfate group () is added to a tyrosine residue of a protein molecule. Secreted proteins and extracellular parts of membrane proteins that pass through the Golgi apparatus may be sulfated. Sulfation was first discovered by Bettelheim in bovine fibrinopeptide B in 1954 and later found to be present in animals and plants but not in prokaryotes or in yeast. Function Sulfation plays a role in strengthening protein-protein interactions. Types of human proteins known to undergo tyrosine sulfation include adhesion molecules, G-protein-coupled receptors, coagulation factors, serine protease inhibitors, extracellular matrix proteins, and hormones. Tyrosine O-sulfate is a stable molecule and is excreted in urine in animals. No enzymatic mechanism of tyrosine sulfate desulfation is known to exist. By knock-out of TPST genes in mice, it may be observed that tyrosine sulfation has effects on the growth of the mice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all List of life sciences, areas of the life sciences are being uncovered and developed through biochemical methodology and research.#Voet, Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis that allows biomolecule, biological molecules to give rise to the processes that occur within living Cell (biology), cells and between cells,#Karp, Karp (2009), p. 2. in turn relating greatly to the understanding of tissue (biology), tissues and organ (anatomy), organs as well as organism structure and function.#Miller, Miller (2012). p. 62. Biochemistry is closely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Protease Inhibitor
Serpins are a superfamily of proteins with similar structures that were first identified for their protease inhibition activity and are found in all kingdoms of life. The acronym serpin was originally coined because the first serpins to be identified act on chymotrypsin-like serine proteases (serine protease inhibitors). They are notable for their unusual mechanism of action, in which they irreversibly inhibit their target protease by undergoing a large conformational change to disrupt the target's active site. This contrasts with the more common competitive mechanism for protease inhibitors that bind to and block access to the protease active site. Protease inhibition by serpins controls an array of biological processes, including coagulation and inflammation, and consequently these proteins are the target of medical research. Their unique conformational change also makes them of interest to the structural biology and protein folding research communities. The conformationa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Biological Chemistry
The ''Journal of Biological Chemistry'' (''JBC'') is a weekly peer-reviewed scientific journal that was established in 1905., jbc.org Since 1925, it is published by the American Society for Biochemistry and Molecular Biology. It covers research in areas of biochemistry and molecular biology. The editor is Alex Toker. the journal is fully open access. In press articles are available free on its website immediately after acceptance. Editors The following individuals have served as editors of the journal: * 1906–1909: John Jacob Abel and Christian Archibald Herter * 1909–1910: Christian Archibald Herter * 1910–1914: Alfred Newton Richards * 1914–1925: Donald D. Van Slyke * 1925–1936: Stanley R. Benedict. After Benedict died, John T. Edsall served as temporary editor until the next editor was appointed. * 1937–1958: Rudolph J. Anderson * 1958–1967: John T. Edsall * 1968–1971: William Howard Stein * 1971–2011: Herbert Tabor * 2011–2015: Martha Fedor * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Von Willebrand Factor
Von Willebrand factor (VWF) () is a blood glycoprotein that promotes primary hemostasis, specifically, platelet adhesion. It is deficient and/or defective in von Willebrand disease and is involved in many other diseases, including thrombotic thrombocytopenic purpura, Heyde's syndrome, and possibly hemolytic–uremic syndrome. Increased plasma levels in many cardiovascular, neoplastic, metabolic (e.g. diabetes), and connective tissue diseases are presumed to arise from adverse changes to the endothelium, and may predict an increased risk of thrombosis. Biochemistry Synthesis VWF is a large multimeric glycoprotein present in blood plasma and produced constitutively as ultra-large VWF in endothelium (in the Weibel–Palade bodies) and megakaryocytes (α-granules of platelets). Structure VWF is synthesized as a prepropeptide comprising 2813 amino acids in endothelial cells and megakaryocytes. The prepropeptide includes a 22-amino acid signal peptide (SP), a 741-amino acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor VIII
Coagulation factor VIII (Factor VIII, FVIII, also known as anti-hemophilic factor (AHF)) is an essential blood clotting protein. In humans, it is encoded by ''F8'' gene. Defects in this gene result in hemophilia A, an X-linked bleeding disorder. Factor VIII is produced in the liver's liver sinusoid, sinusoidal cells and endothelial cells outside the liver throughout the body. This protein circulates in the bloodstream in an inactive form, bound to another molecule called von Willebrand factor, until an injury that damages blood vessels occurs. In response to injury, coagulation factor VIII is activated and separates from von Willebrand factor. The active protein (sometimes written as coagulation factor VIIIa) interacts with another coagulation factor called factor IX. This interaction sets off a chain of additional chemical reactions that form a blood clot. Factor VIII participates in blood coagulation; it is a cofactor for factor IXa, which, in the presence of Ca2+ and phosph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PROSITE
PROSITE is a protein database. It consists of entries describing the protein families, domains and functional sites as well as amino acid patterns and profiles in them. These are manually curated by a team of the Swiss Institute of Bioinformatics and tightly integrated into Swiss-Prot protein annotation. PROSITE was created in 1988 by Amos Bairoch, who directed the group for more than 20 years. Since July 2018, the director of PROSITE and Swiss-Prot is Alan Bridge. PROSITE's uses include identifying possible functions of newly discovered proteins and analysis of known proteins for previously undetermined activity. Properties from well-studied genes can be propagated to biologically related organisms, and for different or poorly known genes biochemical functions can be predicted from similarities. PROSITE offers tools for protein sequence analysis and motif detection (see sequence motif, PROSITE patterns). It is part of the ExPASy proteomics analysis servers. The database ProR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3'-phosphoadenosine-5'-phosphosulfate
3′-Phosphoadenosine-5′-phosphosulfate (PAPS) is a derivative of adenosine monophosphate (AMP) that is phosphorylated at the 3′ position and has a sulfate group attached to the 5′ phosphate. It is the most common coenzyme in sulfotransferase reactions and hence part of sulfation pathways. It is endogenously synthesized by organisms via the phosphorylation of adenosine 5′-phosphosulfate (APS), an intermediary metabolite. In humans such reaction is performed by bifunctional 3′-phosphoadenosine 5′-phosphosulfate synthases ( PAPSS1 and PAPSS2) using ATP as the phosphate donor. Formation and reduction APS and PAPS are intermediates in the reduction of sulfate to sulfite, an exothermic conversion that is carried out by sulfate-reducing bacteria. In these organisms, sulfate serves as an electron acceptor, akin to the use of O2 as an electron acceptor by aerobic organisms. Sulfate is not reduced directly but must be activated by the formation of APS or PAPS. These carrier ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosylprotein Sulfotransferase
Tyrosylprotein sulfotransferase is an enzyme that catalyzes tyrosine sulfation. Function Tyrosylprotein sulfotransferase is the enzyme that catalyzes the sulfation reaction of protein tyrosines, a post-translational modification of proteins. It utilizes 3'-Phosphoadenosine-5'-phosphosulfate (PAPS) as the sulfonate donor and binds proteins with target tyrosine residues to eventually form the tyrosine O-sulfate ester group and the desulfonated 3’-phosphoadenosine-5’-phosphate (PAP). TPST and tyrosine sulfation is involved in a large number of biological and physiological processes. Tyrosine sulfation has been found to be an important part of the inflammatory process, leukocyte movement and cytosis, viral cell entrance, and other cell-cell and protein-protein interactions. Selection for specific tyrosine residues requires a generally accessible tyrosine residue, and acidic residues within +5 or -5 residues of the target tyrosine. P-selectin glycoprotein ligand-1 (PSGL-1) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prokaryote
A prokaryote (; less commonly spelled procaryote) is a unicellular organism, single-celled organism whose cell (biology), cell lacks a cell nucleus, nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Greek (), meaning 'before', and (), meaning 'nut' or 'kernel'. In the earlier two-empire system arising from the work of Édouard Chatton, prokaryotes were classified within the empire Prokaryota. However, in the three-domain system, based upon molecular phylogenetics, prokaryotes are divided into two domain (biology), domains: Bacteria and Archaea. A third domain, Eukaryote, Eukaryota, consists of organisms with nuclei. Prokaryotes evolution, evolved before eukaryotes, and lack nuclei, mitochondria, and most of the other distinct organelles that characterize the eukaryotic cell. Some unicellular prokaryotes, such as cyanobacteria, form colony (biology), colonies held together by biofilms, and large colonies can create multilayered microbial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Posttranslational Modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can expand the chemical set of the 22 amino acids by changing an existing functional group or adding a new one such as phosphate. Phosphorylation is highly effective for controlling the enzyme activity and is the most common change after translation. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosylation, which can promote protein folding and improve stability a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibrinopeptide B
The fibrinopeptides, fibrinopeptide A (FpA) and fibrinopeptide B (FpB), are peptides which are located in the central region of the fibrous glycoprotein fibrinogen (factor I) and are cleaved by the enzyme thrombin (factor IIa) to convert fibrinogen into covalently-linked fibrin (factor IA) monomers. The N-terminal FpA is cleaved from the Aα chains of fibrinogen and FpB from the Bβ chains of fibrinogen, with FpA released before FpB. Subsequent to their formation, fibrin monomers are converted to cross-linked fibrin polymers by the action of thrombin-activated factor XIII (fibrin stabilizing factor), and these fibrin polymers form the backbone of a thrombus (blood clot). Hence, the fibrinopeptides are sensitive markers of fibrinogenesis (fibrin generation), thrombin activity, and coagulation. FpA is a 16-amino acid peptide. The half-life of FpA is very short at approximately 3 to 5 minutes. Hence, FpA levels provide a relatively transient measure of coagulation activa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frederick Bettelheim
Frederick may refer to: People * Frederick (given name), the name Given name Nobility = Anhalt-Harzgerode = *Frederick, Prince of Anhalt-Harzgerode (1613–1670) = Austria = * Frederick I, Duke of Austria (Babenberg), Duke of Austria from 1195 to 1198 * Frederick II, Duke of Austria (1219–1246), last Duke of Austria from the Babenberg dynasty * Frederick the Fair (Frederick I of Austria (Habsburg), 1286–1330), Duke of Austria and King of the Romans = Baden = * Frederick I, Grand Duke of Baden (1826–1907), Grand Duke of Baden * Frederick II, Grand Duke of Baden (1857–1928), Grand Duke of Baden = Bohemia = * Frederick, Duke of Bohemia (died 1189), Duke of Olomouc and Bohemia = Britain = * Frederick, Prince of Wales Frederick, Prince of Wales (Frederick Louis, German: ''Friedrich Ludwig''; 31 January 1707 – 31 March 1751) was the eldest son and heir apparent of King George II of Great Britain. He grew estranged from his parents, King George and Queen C ... ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |