|
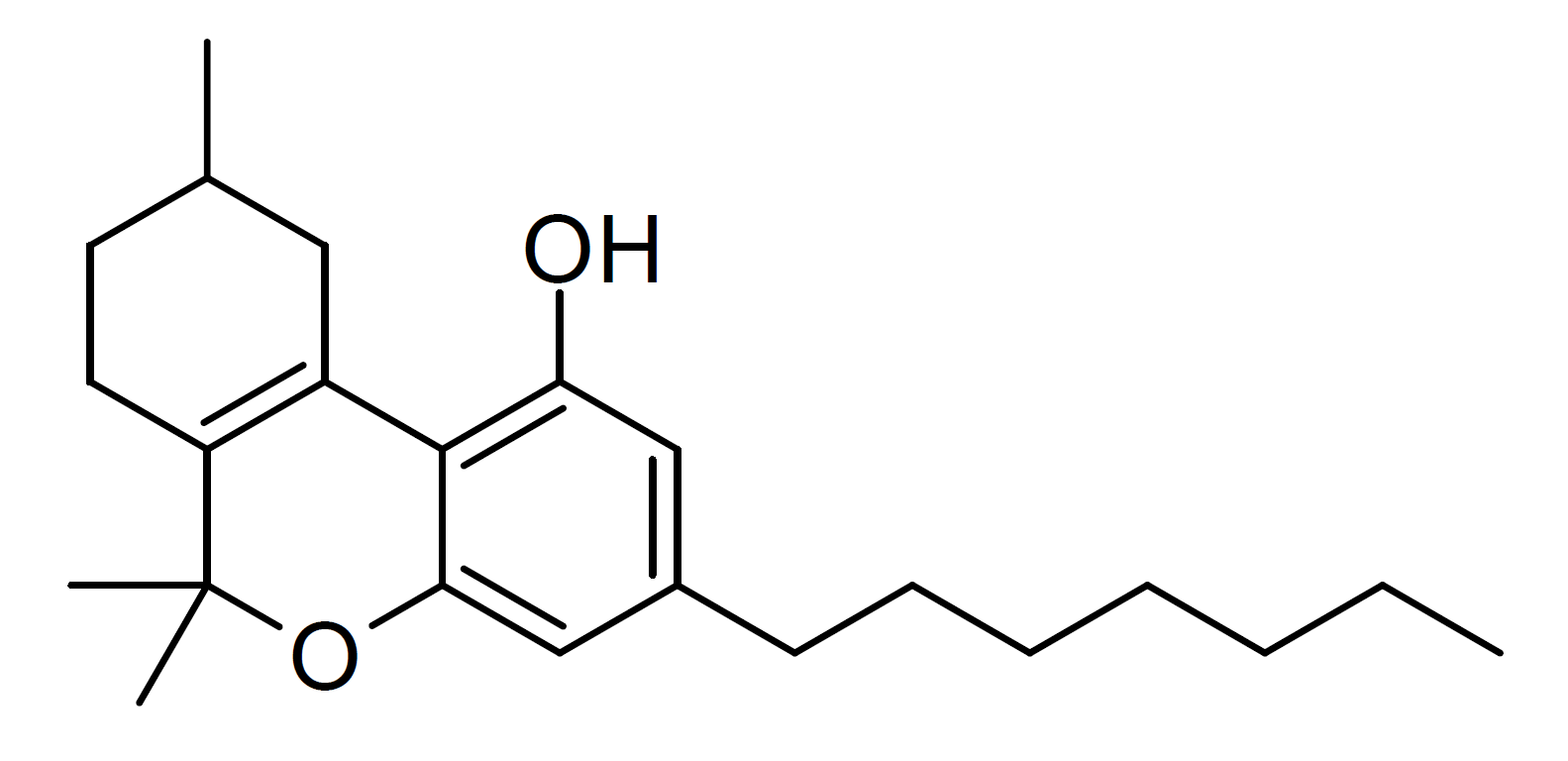
THCP-O-acetate
THCP-O-acetate (THCP-O) is a semi-synthetic derivative of tetrahydrocannabiphorol (THCP) derived by acetylation of the OH group. It has been found as a component of grey-market cannabis products such as e-cigarette liquids and edible gummy lollies, and is allegedly a potent and long-lasting psychoactive cannabinoid. Toxicity In 2022, researchers at Portland State University who screened for the presence of reacted ketene as N-benzylacetamide reported that Vitamin E acetate, CBD-acetate, CBN-acetate and THC-O-acetate may break down to release ketene gas when heated at . Legality Japan banned THCP-O-Acetate along with HHCP on December 26, 2023. See also * Tetrahydrocannabiphorol * Hexahydrocannabiphorol (HHCP) * HHCP-O-acetate * THC-O-acetate * Dimethylheptylpyran (DMHP) * HU-210 * THC-O-phosphate * THC hemisuccinate * THC morpholinylbutyrate THC morpholinylbutyrate (SP-111, Δ9-THC-O- -(morpholin-4-yl)butyrate'') is a synthetic derivative of tetrahydrocannabinol, dev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabiphorol
Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a Cannabinoid receptor type 1, CB1 and Cannabinoid receptor type 2, CB2 agonist, receptor agonist which was known as a synthetic homologue of tetrahydrocannabinol (THC), but for the first time in 2019 was isolated as a natural product in trace amounts from ''Cannabis sativa''. THCP is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." Tetrahydrocannabiphorol has a reported binding affinity of 1.2 nM at CB1, approximately 33 times that of Δ9-THC (40 nM at CB1), however this does not mean it's 33x stronger per milligram. THCP was studied by Roger Adams as early as 1942. Isomers Delta-3-THCP ] The Δ3/Δ6a(10a) isomer Δ3-THCP was synthesised in 1941, and was found to have around the same potency as Delta-3-Tetrahydrocannabinol, Δ3-THC, unl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylheptylpyran
Dimethylheptylpyran (DMHP) is a synthetic cannabinoid and analogue of tetrahydrocannabinol (THC). It was invented in 1949 during attempts to elucidate the structure of Δ9-THC, one of the active components of cannabis. DMHP is a pale yellow, viscous oil which is insoluble in water but dissolves in alcohol or non-polar solvents. DMHP is similar in structure to THC, differing only in the position of one double bond, and the replacement of the 3-pentyl chain with a 3-(1,2-dimethylheptyl) chain. Effects DMPH produces similar activity to THC, such as sedative effects, but is considerably more potent, especially having much stronger analgesic and anticonvulsant effects than THC, although comparatively weaker psychological effects. Mechanism of action It is thought to act as a CB1 receptor agonist, in a similar manner to other cannabinoid derivatives. While DMHP itself has been subject to relatively little study since the characterization of the cannabinoid receptors, the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
THC-O-acetate
THC-O-acetate (THC acetate ester, O-acetyl-THC, THC-O, AcO-THC) is the acetate ester of THC. The term ''THC-O-acetate'' and its variations are commonly used for two types of the substance, dependent on which cannabinoid it is synthesized from. The difference between Δ8-THC and Δ9-THC is the location of the double bond within the cyclohexene ring system. Physical data, chemistry, and properties THC acetate ester (THC-O or THCOA) can be synthesized from THC, or from THCA. The acetylation of THC does not change the properties of the compound to the same extent as with other acetate esters, as the parent compound (THC) is already highly lipophilic, but potency is nonetheless increased to some extent. While the acetate ester of Δ9-THC is the best studied, the acetate esters of other isomers, especially Δ8-THC but also Δ10-THC are also known, as are other esters such as THC-O-propionate, THC-O-phosphate, THC hemisuccinate, THC hemiglutarate, THC morpholinylbutyrate, THC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexahydrocannabiphorol
Hexahydrocannabiphorol (HHCP, sometimes mistakenly referred to as ''hexahydroxycannabiphorol'') is a semi-synthetic cannabinoid derivative which has been marketed since around 2021. It is believed to be made from the hydrogenation of tetrahydrocannabiphorol (THCP). THCP is only reported as a trace component of cannabis in 2019. HHCP was studied by Roger Adams as early as 1942. Pharmacology HHC-P is a partial agonist of the CB1 receptors with an EC50 of 44.4nM for 9R-HHCP and 134nM for 9S-HHCP. Compared to Hexahydrocannabinol (HHC) with an EC50 of 101nM for 9R-HHC and 1,190nM for 9S-HHC In 2021, HHC-P was positively identified in multiple gray market cannabis products in the United States. Legality The legal status of hexahydrocannabinol and derivatives varies between countries, leading to widespread sale in some parts of Europe and the US. In France, HHCP was banned in 2023. In Japan, Japanese Health Ministry announced that six synthetic cannabinoids with structures similar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HHCP-O-acetate
HHCP-O-acetate (HHCPO, HHCP-O) is a semi-synthetic derivative of tetrahydrocannabiphorol (THCP) derived in several steps by hydrogenation to hexahydrocannabiphorol (HHCP) followed by acetylation of the OH group. It has been found as a component of grey-market cannabis products such as e-cigarette liquids and edible gumdrops, and is allegedly a potent and long-lasting psychoactive cannabinoid. Confusion about acronym Some vendors have mistakenly claimed that HHCPO stands for hexahydrocannabinol propionate (HHC-P) or hexahydrocannabinol cyclopropyl ether, but these are different compounds. : See also * Abeo-HHC acetate * Hexahydrocannabinol * Hexahydrocannabihexol * THC-O-acetate * THCP-O-acetate THCP-O-acetate (THCP-O) is a semi-synthetic derivative of tetrahydrocannabiphorol (THCP) derived by acetylation of the OH group. It has been found as a component of grey-market cannabis products such as e-cigarette liquids and edible gummy lolli ... References Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HHCP
Hexahydrocannabiphorol (HHCP, sometimes mistakenly referred to as ''hexahydroxycannabiphorol'') is a semi-synthetic cannabinoid derivative which has been marketed since around 2021. It is believed to be made from the hydrogenation of tetrahydrocannabiphorol (THCP). THCP is only reported as a trace component of cannabis in 2019. HHCP was studied by Roger Adams as early as 1942. Pharmacology HHC-P is a partial agonist of the CB1 receptors with an EC50 of 44.4nM for 9R-HHCP and 134nM for 9S-HHCP. Compared to Hexahydrocannabinol (HHC) with an EC50 of 101nM for 9R-HHC and 1,190nM for 9S-HHC In 2021, HHC-P was positively identified in multiple gray market cannabis products in the United States. Legality The legal status of hexahydrocannabinol and derivatives varies between countries, leading to widespread sale in some parts of Europe and the US. In France, HHCP was banned in 2023. In Japan, Japanese Health Ministry announced that six synthetic cannabinoids with structures similar t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
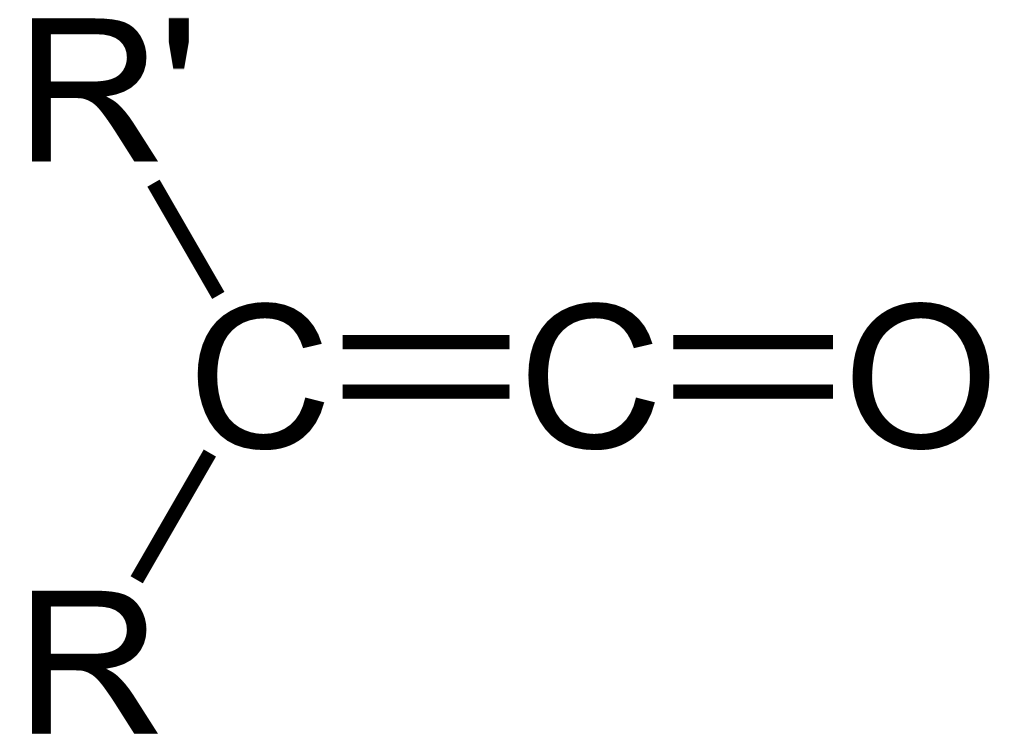
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinol
Cannabinol (CBN) is a mildly psychoactive phytocannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS). Although CBN shares the same mechanism of action as other phytocannabinoids (e.g., Delta-9-tetrahydrocannabinol, Δ9-THC), it has a lower affinity for CB1 receptors, meaning that much higher doses of CBN are required in order to experience effects, such as mild sedation. It was the first cannabinoid to be isolated from cannabis and was discovered in 1896. Pharmacology Pharmacodynamics Both THC and CBN activate the CB1 (Ki = 211.2 nM) and CB2 (Ki = 126.4 nM) receptors. Each compound acts as a low affinity partial agonist at CB1 receptors with THC demonstrating 5x–10× greater affinity to the CB1 receptor. Like THC, CBN has a higher selectivity for CB2 receptors which are located throughout the central and peripheral nerv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylation
: In chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply ''acetates''. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Acetylation/deacetylation in biology Histone deacetylases "play crucial roles in gene transcription and most likely in all eukaryotic biological processes that involve chromatin". Acetylation is one type of post-translational modification of proteins. The acetylation of the ε-amino group of lysine, which is common, converts a charged side chain to a neutral one. Acetylation/deacetylation of histones also plays a role in gene expression and cancer. These modifications are effected by enzymes called histone acetyltransferases (HATs) and histone deacetylases (HDACs). Two general mechanisms are known for deacetylation. One mechanism involves zinc binding to the acetyl o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabidiol
Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in ''Cannabis'', along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. Medically, it is an anticonvulsant used to treat multiple forms of epilepsy. It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient evidence-based medicine, high-quality evidence that CBD is effective for these conditions. CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects. Cannabidiol can be route of administration, taken internally in multiple ways, including by Route of administration#Inhalation, inhaling cannabis cannabis smoking, smoke or vaporizer (inhalation device), vapor, Oral administration, swallowing it by mouth, and through use of an aerosol spray into the buccal administration, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |