|
Shikimate O-hydroxycinnamoyltransferase
In enzymology, a shikimate O-hydroxycinnamoyltransferase () is an enzyme that catalyzes the chemical reaction :4-coumaroyl-CoA + shikimate \rightleftharpoons CoA + 4-coumaroylshikimate Thus, the two substrates of this enzyme are 4-coumaroyl-CoA and shikimate, whereas its two products are CoA and 4-coumaroylshikimate. This enzyme belongs to the family of transferases, specifically those acyltransferases transferring groups other than aminoacyl groups. The systematic name of this enzyme class is 4-coumaroyl-CoA:shikimate O-(hydroxycinnamoyl)transferase. This enzyme is also called shikimate hydroxycinnamoyltransferase. This enzyme participates in phenylpropanoid biosynthesis The biosynthesis of phenylpropanoids involves a number of enzymes. From amino acids to cinnamates In plants, all phenylpropanoids are derived from the amino acids phenylalanine and tyrosine. Phenylalanine ammonia-lyase (PAL, a.k.a. phenylalanine .... References * * EC 2.3.1 Enzymes of unkn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzymology
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytochemistry (journal)
''Phytochemistry'' is a peer-reviewed scientific journal covering pure and applied plant chemistry, plant biochemistry and molecular biology. It is published by Elsevier and is an official publication for the Phytochemical Society of Europe, the Phytochemical Society of North America, and the Phytochemical Society of Asia. A sister journal ''Phytochemistry Letters'' is published since 2008. Abstracting and indexing ''Phytochemistry'' is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 4.072. References External links {{Official website, http://www.journals.elsevier.com/phytochemistry/ Biochemistry journals Botany journals Elsevier academic journals En ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
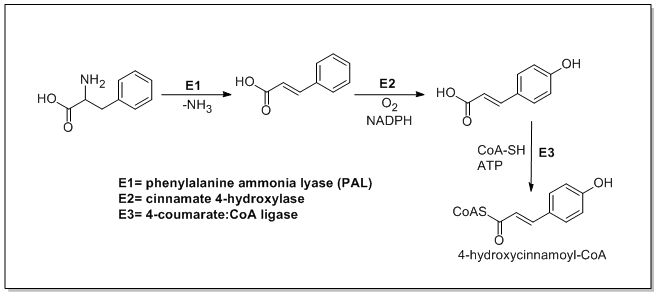
Phenylpropanoid Biosynthesis
The biosynthesis of phenylpropanoids involves a number of enzymes. From amino acids to cinnamates In plants, all phenylpropanoids are derived from the amino acids phenylalanine and tyrosine. Phenylalanine ammonia-lyase (PAL, a.k.a. phenylalanine/tyrosine ammonia-lyase) is an enzyme that transforms L-phenylalanine and tyrosine into trans-cinnamic acid and ''p''-coumaric acid, respectively. Trans-cinnamate 4-monooxygenase (cinnamate 4-hydroxylase) is the enzyme that transforms trans-cinnamate into 4-hydroxycinnamate (''p''-coumaric acid). 4-Coumarate-CoA ligase is the enzyme that transforms 4-coumarate (''p''-coumaric acid) into 4-coumaroyl-CoA. Enzymes associated with biosynthesis of hydroxycinnamic acids * Cinnamyl-alcohol dehydrogenase (CAD), an enzyme that transforms cinnamyl alcohol into cinnamaldehyde * Sinapine esterase, an enzyme that transforms sinapoylcholine into sinapate ( sinapic acid) and choline * Trans-cinnamate 2-monooxygenase, an enzyme that transforms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Enzymes
This article lists enzymes by their classification in the International Union of Biochemistry and Molecular Biology's Enzyme Commission (EC) numbering system. * List of EC numbers (EC 5) * List of EC numbers (EC 6) :Oxidoreductases (EC 1) ( Oxidoreductase) * Dehydrogenase *Luciferase * DMSO reductase :EC 1.1 (act on the CH-OH group of donors) * :EC 1.1.1 (with NAD+ or NADP+ as acceptor) ** Alcohol dehydrogenase (NAD) ** Alcohol dehydrogenase (NADP) ** Homoserine dehydrogenase ** Aminopropanol oxidoreductase **Diacetyl reductase ** Glycerol dehydrogenase ** Propanediol-phosphate dehydrogenase **glycerol-3-phosphate dehydrogenase (NAD+) **D-xylulose reductase ** L-xylulose reductase ** Lactate dehydrogenase ** Malate dehydrogenase ** Isocitrate dehydrogenase **HMG-CoA reductase * :EC 1.1.2 (with a cytochrome as acceptor) * :EC 1.1.3 (with oxygen as acceptor) ** Glucose oxidase ** L-gulonolactone oxidase ** Thiamine oxidase ** Xanthine oxidase * :EC 1.1. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyltransferase
Acyltransferase is a type of transferase enzyme that acts upon acyl groups. Examples include: * Glyceronephosphate O-acyltransferase * Lecithin-cholesterol acyltransferase *Long-chain-alcohol O-fatty-acyltransferase In enzymology, a long-chain-alcohol O-fatty-acyltransferase () is an enzyme that catalyzes the chemical reaction :acyl-CoA + a long-chain alcohol \rightleftharpoons CoA + a long-chain ester Thus, the two substrates of this enzyme are acyl-Co ... See also * Acetyltransferase External links * Transferases EC 2.3 {{2.3-enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferase
A transferase is any one of a class of enzymes that catalyse the transfer of specific functional groups (e.g. a methyl or glycosyl group) from one molecule (called the donor) to another (called the acceptor). They are involved in hundreds of different biochemical pathways throughout biology, and are integral to some of life's most important processes. Transferases are involved in myriad reactions in the cell. Three examples of these reactions are the activity of coenzyme A (CoA) transferase, which transfers thiol esters, the action of N-acetyltransferase, which is part of the pathway that metabolizes tryptophan, and the regulation of pyruvate dehydrogenase (PDH), which converts pyruvate to acetyl CoA. Transferases are also utilized during translation. In this case, an amino acid chain is the functional group transferred by a peptidyl transferase. The transfer involves the removal of the growing amino acid chain from the tRNA molecule in the A-site of the ribosome a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenate (vitamin B5), and adenosine triphosphate (ATP). In its acetyl form, coenzyme A is a highly versatile molecule, serving metabolic functions in both the anabolic and catabolic pathways. Acetyl-CoA is utilised in the post-translational regulation and allosteric regulation of pyruvate dehydrogenase and carboxylase to maintain and support the partition of pyruvate synthesis and degradation. Discovery of structure Coenzyme A was identified by Fritz Lipmann in 1946, who also later gave it its name. Its structure was determined during the early 1950s at the Lister Institute, London, together by L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are Ribozyme, catalytic RNA molecules, called ribozymes. Enzymes' Chemical specificity, specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Product (chemistry)
Products are the species formed from chemical reactions. During a chemical reaction, reactants are transformed into products after passing through a high energy transition state. This process results in the consumption of the reactants. It can be a spontaneous reaction or mediated by catalysts which lower the energy of the transition state, and by solvents which provide the chemical environment necessary for the reaction to take place. When represented in chemical equations, products are by convention drawn on the right-hand side, even in the case of reversible reactions. The properties of products such as their energies help determine several characteristics of a chemical reaction, such as whether the reaction is exergonic or endergonic. Additionally, the properties of a product can make it easier to extract and purify following a chemical reaction, especially if the product has a different state of matter than the reactants. Spontaneous reaction : R \rightarrow P *Where ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shikimate
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower ''shikimi'' (, the Japanese star anise, ''Illicium anisatum''), from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later. Biosynthesis Phosphoenolpyruvate and erythrose-4-phosphate condense to form 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP), in a reaction catalyzed by the enzyme DAHP synthase. DAHP is then transformed to 3-dehydroquinate (DHQ), in a reaction catalyzed by DHQ synthase. Although this reaction requires nicotinamide adenine dinucleotide (NAD) as a cofactor, the enzymic mechanism regenerates it, resulting in the net use of no NAD. : DHQ is dehydrated to 3-dehydroshikimic acid by the enzyme 3-dehydroquinate dehydratase, which is reduced to shi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |