|
Shikimate
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower ''shikimi'' (, the Japanese star anise, ''Illicium anisatum''), from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later. Biosynthesis Phosphoenolpyruvate and erythrose-4-phosphate condense to form 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP), in a reaction catalyzed by the enzyme DAHP synthase. DAHP is then transformed to 3-dehydroquinate (DHQ), in a reaction catalyzed by DHQ synthase. Although this reaction requires nicotinamide adenine dinucleotide (NAD) as a cofactor, the enzymic mechanism regenerates it, resulting in the net use of no NAD. : DHQ is dehydrated to 3-dehydroshikimic acid by the enzyme 3-dehydroquinate dehydratase, which is reduced to shi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shikimate Dehydrogenase
In enzymology, a shikimate dehydrogenase () is an enzyme that catalysis, catalyzes the chemical reaction :shikimate + NADP+ \rightleftharpoons 3-dehydroshikimate + NADPH + H+ Thus, the two substrate (biochemistry), substrates of this enzyme are shikimate and nicotinamide adenine dinucleotide phosphate, NADP+, whereas its 3 product (chemistry), products are 3-dehydroshikimate, nicotinamide adenine dinucleotide phosphate, NADPH, and hydrogen ion, H+. This enzyme participates in phenylalanine, tyrosine and tryptophan biosynthesis. Function Shikimate dehydrogenase is an enzyme that catalyzes one step of the shikimic acid, shikimate pathway. This pathway is found in bacteria, plants, fungi, algae, and parasites and is responsible for the biosynthesis of aromatic amino acids (phenylalanine, tyrosine, and tryptophan) from the metabolism of carbohydrates. In contrast, animals and humans lack this pathway hence products of this biosynthetic route are essential amino acids that must be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DAHP Synthase
3-Deoxy-D-arabinoheptulosonate 7-phosphate (DAHP) synthase () is the first enzyme in a series of metabolic Chemical reaction, reactions known as the shikimate pathway, which is responsible for the biosynthesis of the amino acids phenylalanine, tyrosine, and tryptophan. Since it is the first enzyme in the shikimate pathway, it controls the amount of carbon entering the pathway. Enzyme enzyme inhibitor, inhibition is the primary method of regulating the amount of carbon entering the pathway. Forms of this enzyme differ between organisms, but can be considered DAHP synthase based upon the reaction that is catalyzed by this enzyme. In enzymology, a DAHP synthase () is an enzyme that catalysis, catalyzes the chemical reaction :phosphoenolpyruvate + D-erythrose 4-phosphate + H2O \rightleftharpoons 3-deoxy-D-arabino-hept-2-ulosonate 7-phosphate + phosphate The three substrate (biochemistry), substrates of this enzyme are phosphoenolpyruvate, D-erythrose 4-phosphate, and water, H2O, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shikimate Pathway 2
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower ''shikimi'' (, the Japanese star anise, ''Illicium anisatum''), from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later. Biosynthesis Phosphoenolpyruvate and erythrose-4-phosphate condense to form 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP), in a reaction catalyzed by the enzyme DAHP synthase. DAHP is then transformed to 3-dehydroquinate (DHQ), in a reaction catalyzed by DHQ synthase. Although this reaction requires nicotinamide adenine dinucleotide (NAD) as a cofactor, the enzymic mechanism regenerates it, resulting in the net use of no NAD. : DHQ is dehydrated to 3-dehydroshikimic acid by the enzyme 3-dehydroquinate dehydratase, which is reduced to shiki ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Amino Acid
An aromatic amino acid is an amino acid that includes an aromaticity, aromatic ring. Among the 20 standard amino acids, histidine, phenylalanine, tryptophan, tyrosine, are classified as aromatic. Properties and function Optical properties Aromatic amino acids, excepting histidine, absorb Ultraviolet, ultraviolet light above and beyond 250 nm and will fluorescence, fluoresce under these conditions. This characteristic is used in quantitative analysis, notably in determining the concentrations of these amino acids in solution. Most proteins Absorption spectroscopy, absorb at 280 nm due to the presence of tyrosine and tryptophan. Of the aromatic amino acids, tryptophan has the highest extinction coefficient; its absorption maximum occurs at 280 nm. The absorption maximum of tyrosine occurs at 274 nm. Role in protein structure and function Aromatic amino acids stabilize folded structures of many proteins. Aromatic residues are found predominantly sequestered within the cores o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-dehydroquinate Dehydratase
The enzyme 3-dehydroquinate dehydratase () catalyzes the chemical reaction :3-dehydroquinate \rightleftharpoons 3-dehydroshikimate + H2O This enzyme belongs to the family of lyases, specifically the hydro-lyases, which cleave carbon-oxygen bonds. This enzyme participates in phenylalanine, tyrosine and tryptophan biosynthesis. Discovery The shikimate pathway was determined to be a major biosynthetic route for the production of aromatic amino acids through the research of Bernhard Davis and David Sprinson. Role in the shikimate pathway 3-Dehydroquinate Dehydratase is an enzyme that catalyzes the third step of the shikimate pathway. The shikimate pathway is a biosynthetic pathway that allows plants, fungi, and bacteria to produce aromatic amino acids. Mammals do not have this pathway, meaning that they must obtain these essential amino acids through their diet. Aromatic Amino acids include Phenylalanine, Tyrosine, and Tryptophan. This enzyme dehydrates 3-Dehydroquinate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
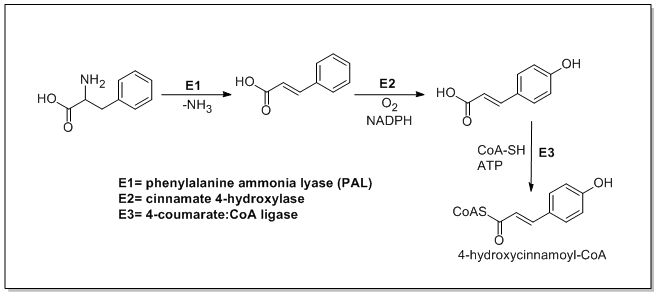
Phenylpropanoid Biosynthesis
The biosynthesis of phenylpropanoids involves a number of enzymes. From amino acids to cinnamates In plants, all phenylpropanoids are derived from the amino acids phenylalanine and tyrosine. Phenylalanine ammonia-lyase (PAL, a.k.a. phenylalanine/tyrosine ammonia-lyase) is an enzyme that transforms L-phenylalanine and tyrosine into trans-cinnamic acid and p-coumaric acid, ''p''-coumaric acid, respectively. Trans-cinnamate 4-monooxygenase (cinnamate 4-hydroxylase) is the enzyme that transforms trans-cinnamate into 4-hydroxycinnamate (''p''-coumaric acid). 4-Coumarate-CoA ligase is the enzyme that transforms 4-coumarate (''p''-coumaric acid) into 4-coumaroyl-CoA. Enzymes associated with biosynthesis of hydroxycinnamic acids * Cinnamyl-alcohol dehydrogenase (CAD), an enzyme that transforms cinnamyl alcohol into cinnamaldehyde * Sinapine esterase, an enzyme that transforms sinapoylcholine into sinapate (sinapic acid) and choline * Trans-cinnamate 2-monooxygenase, an enzyme that tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DHQ Synthase
The enzyme 3-dehydroquinate synthase (EC 4.2.3.4) catalyzes the chemical reaction : 3-deoxy-D-''arabino''-hept-2-ulosonate 7-phosphate \rightleftharpoons 3-dehydroquinate + phosphate The protein uses NAD+ to catalyze the reaction. This reaction is part of the shikimate pathway which is involved in the biosynthesis of aromatic amino acids. 3-Dehydroquinate synthase belongs to the family of lyases, to be specific those carbon-oxygen lyases acting on phosphates. This enzyme participates in phenylalanine, tyrosine, and tryptophan biosynthesis. It employs one cofactor, cobalt (Co2+). Background The shikimate pathway is composed of seven steps, each catalyzed by an enzyme. The shikimate pathway is responsible for producing the precursors for aromatic amino acids, which are essential to our diets because we cannot synthesize them in our bodies. Only plants, bacteria, and microbial eukaryotes are capable of producing aromatic amino acids. The pathway ultimately converts phospho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a Heterocyclic compound, heterocyclic ring (C, the ring containing the embedded oxygen). This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature, they can be classified into: *flavonoids or bioflavonoids *isoflavonoids, derived from 3-phenylchromone, chromen-4-one (3-phenyl-1,4-benzopyran, benzopyrone) structure *neoflavonoids, derived from 4-phenylcoumarin (4-phenyl-1,2-benzopyran, benzopyrone) structure The three flavonoid classes above are all ketone-containing compounds and as such, anthoxanthins (flavones and flavonols). This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the chemical formula, formula . It can be viewed as a benzyl group substituent, substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and chemical polarity, nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The chirality (chemistry)#Naming conventions, L-isomer is used to biochemically form proteins coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the biological pigment melanin. It is Genetic code, encoded by the messenger RNA codons UUU and UUC. Phenylalanine is found naturally in the milk of mammals. It is used in the manufacture of food and drink products and sold as a nutritional supplement as it is a direct precursor to the neuromodulation, neuromodulator phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylketonuria
Phenylketonuria (PKU) is an inborn error of metabolism that results in decreased metabolism of the amino acid phenylalanine. Untreated PKU can lead to intellectual disability, seizures, behavioral problems, and mental disorders. It may also result in a musty smell and lighter skin. A baby born to a mother who has poorly treated PKU may have heart problems, a small head, and low birth weight. Phenylketonuria is an inherited genetic disorder. It is caused by mutations in the '' PAH'' gene, which can result in inefficient or nonfunctional phenylalanine hydroxylase, an enzyme responsible for the metabolism of excess phenylalanine. This results in the buildup of dietary phenylalanine to potentially toxic levels. It is autosomal recessive, meaning that both copies of the gene must be mutated for the condition to develop. The two main types are classic PKU and variant PKU, depending on whether any enzyme function remains. Those with one copy of a mutated gene typically do not ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Amino Acid
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life forms, the nine amino acids humans cannot synthesize are valine, isoleucine, leucine, methionine, phenylalanine, tryptophan, threonine, histidine, and lysine. Six other amino acids are considered conditionally essential in the human diet, meaning their synthesis can be limited under special pathophysiological conditions, such as prematurity in the infant or individuals in severe catabolic distress. These six are arginine, cysteine, glycine, glutamine, proline, and tyrosine. Six amino acids are non-essential (dispensable) in humans, meaning they can be synthesized in sufficient quantities in the body. These six are alanine, aspartic acid, asparagine, glutamic acid, serine, and selenocysteine (considered the 21st amino acid). Pyrr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |