|
Sal Hepatica
Sal Hepatica is the name of a mineral salt laxative that was produced and marketed by Bristol-Myers from its inception in 1887, becoming its first nationally recognized product in 1903, until 1958. When dissolved in water, it was said to reproduce the taste and effect of the natural mineral waters of Bohemia. Composition and mechanism of action The product was composed of Glauber's salt (sodium sulfate), baking soda ( sodium bicarbonate), tartaric acid, common salt (sodium chloride), sodium phosphate and traces of lithium carbonate and water. It was marketed as a saline laxative and alkalinizing agent. In the latter role it was recommended for dissolving uric acid in gout and "rheumatism", and for various other stomach, liver, and kidney disorders. See also * Epsom Salt A Magnesium sulfate salt marketed as the active ingredient in the water from Epsom Epsom is the principal town of the Borough of Epsom and Ewell in Surrey, England, about south of central Londo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sal Hepatica By Bristol Myers Co (1909)
Sal, SAL, or S.A.L. may refer to: Personal name * Sal (name), a list of people and fictional characters with the given name or nickname Places * Sal, Cape Verde, an island and municipality * Sal, Iran, a village in East Azerbaijan Province * Cay Sal, a small island between Florida, Cuba and the Bahamas * Sal Glacier, Queen Maud Land, Antarctica * Sal River (India), Goa * Sal (Russia), a tributary of the Don in southern Russia Arts and entertainment * ''Sal'' (film), a 2011 film about Sal Mineo * Laffing Sal, an automated character Science * Sal (tree) (''Shorea robusta''), from the Indian subcontinent * Saharan Air Layer, or SAL * Salivary lipocalin, or SAL, the pig major urinary protein homologue * Society of Antiquaries of London, a British historical and archaeological learned society * Sterility assurance level, or SAL, in microbiology Transportation * Seaboard Air Line Railroad, reporting mark SAL * Sociedade de Aviação Ligeira, or SAL, Luanda, Angola, an air taxi op ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saline Laxative
Laxatives, purgatives, or aperients are substances that loosen stools and increase bowel movements. They are used to treat and prevent constipation. Laxatives vary as to how they work and the side effects they may have. Certain stimulant, lubricant and saline laxatives are used to evacuate the colon for rectal and bowel examinations, and may be supplemented by enemas under certain circumstances. Sufficiently high doses of laxatives may cause diarrhea. Some laxatives combine more than one active ingredient. Laxatives may be administered orally or rectally. Types Bulk-forming agents Bulk-forming laxatives, also known as roughage, are substances, such as fiber in food and hydrophilic agents in over-the-counter drugs, that add bulk and water to stools so that they can pass more easily through the intestines (lower part of the digestive tract). Properties * Site of action: small and large intestines * Onset of action: 12–72 hours * Examples: dietary fiber, Metamucil, Citruc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epsom
Epsom is the principal town of the Borough of Epsom and Ewell in Surrey, England, about south of central London. The town is first recorded as ''Ebesham'' in the 10th century and its name probably derives from that of a Saxon landowner. The earliest evidence of human activity is from the mid-Bronze Age, but the modern settlement probably grew up in the area surrounding St Martin's Church in the 6th or 7th centuries and the street pattern is thought to have become established in the Middle Ages. Today the High Street is dominated by the clock tower, which was erected in 1847–8. Like other nearby settlements, Epsom is located on the spring line where the permeable chalk of the North Downs meets the impermeable London Clay. Several tributaries of the Hogsmill River rise in the town and in the 17th and early 18th centuries, the spring on Epsom Common was believed to have healing qualities. The mineral waters were found to be rich in ''Epsom salts'', which were later ident ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Sulfate
Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, soluble in water but not in ethanol. Magnesium sulfate is usually encountered in the form of a hydrate , for various values of ''n'' between 1 and 11. The most common is the heptahydrate , known as Epsom salt, which is a household chemical with many traditional uses, including bath salts. The main use of magnesium sulfate is in agriculture, to correct soils deficient in magnesium (an essential plant nutrient because of the role of magnesium in chlorophyll and photosynthesis). The monohydrate is favored for this use; by the mid 1970s, its production was 2.3 million tons per year. The anhydrous form and several hydrates occur in nature as minerals, and the salt is a significant component of the water from some springs. Hydrates Magnesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epsom Salt
Epsomite, Epsom salt, or magnesium sulfate heptahydrate, is a hydrous magnesium sulfate mineral with formula MgSO4·7H2O. Epsomite crystallizes in the orthorhombic system as rarely found acicular or fibrous crystals, the normal form is as massive encrustations. It is colorless to white with tints of yellow, green and pink. The Mohs hardness is 2 to 2.5 and it has a low specific gravity of 1.67. It is readily soluble in water. It absorbs water from the air and converts to hexahydrate with the loss of one water molecule and a switch to monoclinic structure. Etymology It was first systematically described in 1806 for an occurrence near Epsom, Surrey, England, after which it was named. Discovery and occurrence Epsomite forms as encrustations or efflorescences on limestone cavern walls and mine timbers and walls, rarely as volcanic fumarole deposits, and as rare beds in evaporite layers such as those found in certain bodies of salt water. It occurs in association with melanteri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rheumatism
Rheumatism or rheumatic disorders are conditions causing chronic, often intermittent pain affecting the joints or connective tissue. Rheumatism does not designate any specific disorder, but covers at least 200 different conditions, including arthritis and "non-articular rheumatism", also known as "regional pain syndrome" or "soft tissue rheumatism". There is a close overlap between the term soft tissue disorder and rheumatism. Sometimes the term "soft tissue rheumatic disorders" is used to describe these conditions. The term "Rheumatic Diseases" is used in MeSH to refer to connective tissue disorders. The branch of medicine devoted to the diagnosis and therapy of rheumatism is called rheumatology. Types Many rheumatic disorders of chronic, intermittent pain (including joint pain, neck pain or back pain) have historically been caused by infectious diseases. Their etiology was unknown until the 20th century and not treatable. Postinfectious arthritis, also known as reactiv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gout
Gout ( ) is a form of inflammatory arthritis characterized by recurrent attacks of a red, tender, hot and swollen joint, caused by deposition of monosodium urate monohydrate crystals. Pain typically comes on rapidly, reaching maximal intensity in less than 12 hours. The joint at the base of the big toe is affected in about half of cases. It may also result in tophi, kidney stones, or kidney damage. Gout is due to persistently elevated levels of uric acid in the blood. This occurs from a combination of diet, other health problems, and genetic factors. At high levels, uric acid crystallizes and the crystals deposit in joints, tendons, and surrounding tissues, resulting in an attack of gout. Gout occurs more commonly in those who: regularly drink beer or sugar-sweetened beverages; eat foods that are high in purines such as liver, shellfish, or anchovies; or are overweight. Diagnosis of gout may be confirmed by the presence of crystals in the joint fluid or in a deposit o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uric Acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of purine nucleotides, and it is a normal component of urine. High blood concentrations of uric acid can lead to gout and are associated with other medical conditions, including diabetes and the formation of ammonium acid urate kidney stones. Chemistry Uric acid was first isolated from kidney stones in 1776 by Swedish chemist Carl Wilhelm Scheele. In 1882, the Ukrainian chemist Ivan Horbaczewski first synthesized uric acid by melting urea with glycine. Uric acid displays lactam–lactim tautomerism (also often described as keto–enol tautomerism). Although the lactim form is expected to possess some degree of aromaticity, uric acid crystallizes in the lactam form, with computational chemistry also indicating that tautomer to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkalinizing Agent
Alkalinizing agents are drugs used to manage disorders associated with low pH. For example, they may be used to treat acidosis due to kidney failure. Used for oral or parenteral therapy, sodium bicarbonate is the commonly preferred alkalinizing agent. Others include potassium citrate, calcium carbonate, sodium lactate and calcium acetate Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous .... References {{treatment-stub Drugs acting on the genito-urinary system ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Carbonate
Lithium carbonate is an inorganic compound, the lithium salt of carbonate with the formula . This white salt is widely used in the processing of metal oxides. It is listed on the World Health Organization's List of Essential Medicines because it can be used as a treatment for mood disorders such as bipolar disorder. Uses Lithium carbonate is an important industrial chemical. Its main use is as a precursor for compounds used in lithium-ion batteries. Glasses derived from lithium carbonate are useful in ovenware. Lithium carbonate is a common ingredient in both low-fire and high-fire ceramic glaze. It forms low-melting fluxes with silica and other materials. Its alkaline properties are conducive to changing the state of metal oxide colorants in glaze, particularly red iron oxide (). Cement sets more rapidly when prepared with lithium carbonate, and is useful for tile adhesives. When added to aluminium trifluoride, it forms LiF which gives a superior electrolyte for the proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laxative
Laxatives, purgatives, or aperients are substances that loosen stools and increase bowel movements. They are used to treat and prevent constipation. Laxatives vary as to how they work and the side effects they may have. Certain stimulant, lubricant and saline laxatives are used to evacuate the colon for rectal and bowel examinations, and may be supplemented by enemas under certain circumstances. Sufficiently high doses of laxatives may cause diarrhea. Some laxatives combine more than one active ingredient. Laxatives may be administered orally or rectally. Types Bulk-forming agents Bulk-forming laxatives, also known as roughage, are substances, such as fiber in food and hydrophilic agents in over-the-counter drugs, that add bulk and water to stools so that they can pass more easily through the intestines (lower part of the digestive tract). Properties * Site of action: small and large intestines * Onset of action: 12–72 hours * Examples: dietary fiber, Metamuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
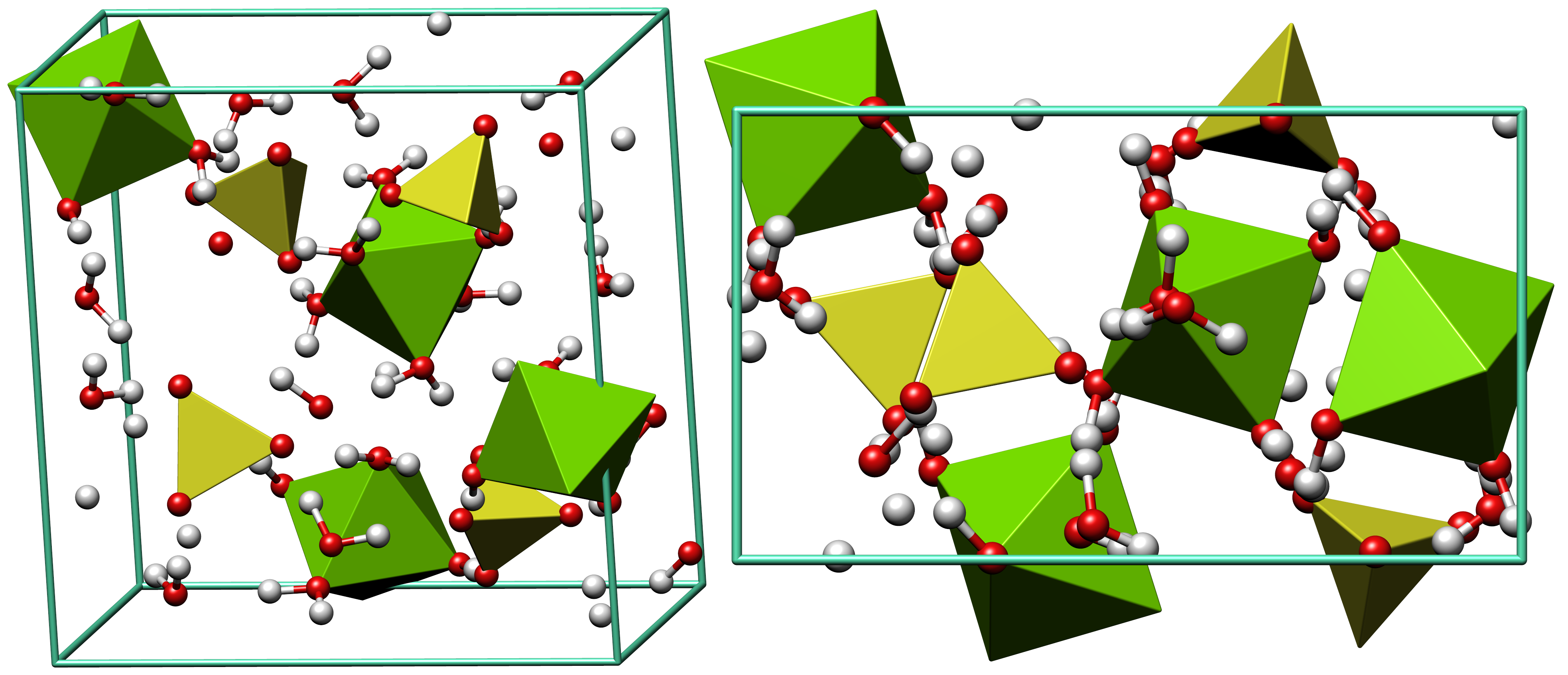
Sodium Phosphate
Sodium phosphate is a generic term for a variety of salts of sodium (Na+) and phosphate (PO43−). Phosphate also forms families or condensed anions including di-, tri-, tetra-, and polyphosphates. Most of these salts are known in both anhydrous (water-free) and hydrated forms. The hydrates are more common than the anhydrous forms. Uses Sodium phosphates have many applications in food and for water treatment. For example, sodium phosphates are often used as emulsifiers (as in processed cheese), thickening agents, and leavening agents for baked goods. They are also used to control pH of processed foods. They are also used in medicine for constipation and to prepare the bowel for medical procedures. Moreover, they are used in detergents for softening water, and as an efficient anti rust solution. Adverse effects Sodium phosphates are popular in commerce in part because they are inexpensive and because they are nontoxic at normal levels of consumption. However, oral sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)

.jpg)