|
Magnesium Sulfate
Magnesium sulfate or magnesium sulphate is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, soluble in water but not in ethanol. Magnesium sulfate is usually encountered in the form of a hydrate , for various values of ''n'' between 1 and 11. The most common is the heptahydrate , known as Epsom salt, which is a household chemical with many traditional uses, including bath salts. The main use of magnesium sulfate is in agriculture, to correct soils deficient in magnesium (an essential plant nutrient because of the role of magnesium in chlorophyll and photosynthesis). The monohydrate is favored for this use; by the mid 1970s, its production was 2.3 million tons per year. The anhydrous form and several hydrates occur in nature as minerals, and the salt is a significant component of the water from some springs. Hydrates Magnesium sulfate can crystallize as several hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
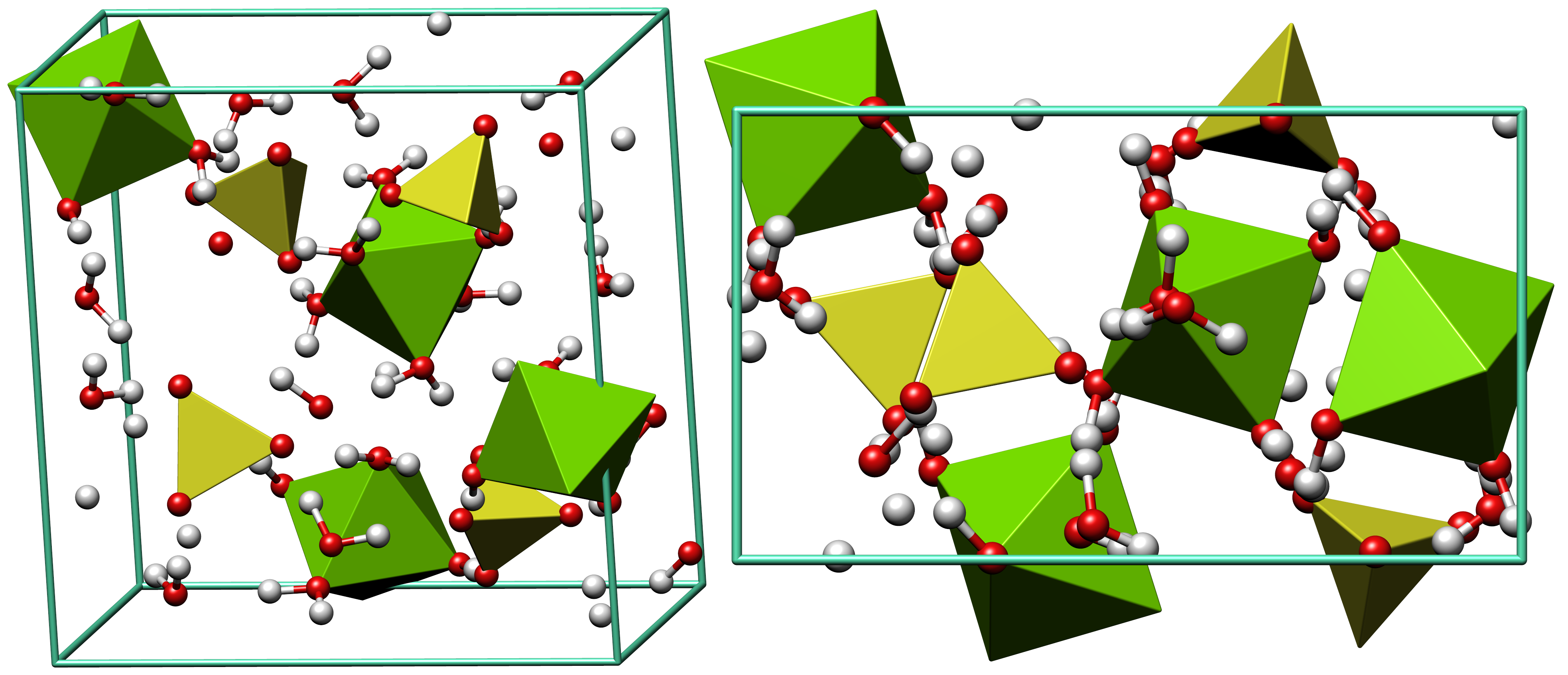
Epsomite
Epsomite, Epsom salt, or magnesium sulfate heptahydrate, is a hydrous magnesium sulfate mineral with formula . Physical properties Epsomite crystallizes in the orthorhombic system. The normal form is as massive encrustations, while acicular or fibrous crystals are rarely found. It is colorless to white with tints of yellow, green and pink. It is a soft mineral with variable Mohs hardness around 2.0~2.5, and it has a low specific gravity It is readily soluble in water, and absorbs water from the air. It converts to hexahydrate with the loss of one water molecule and a switch to monoclinic structure. The epsomite group includes solid solution series with morenosite (·) and goslarite (·). Etymology It was first systematically described in 1806 for an occurrence near Epsom, Surrey, England, after which it was named. It has been also referred to as "cave cotton" when in its fibrous form. Occurrence Epsomite forms as encrustations or efflorescences on limestone caver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epsomite
Epsomite, Epsom salt, or magnesium sulfate heptahydrate, is a hydrous magnesium sulfate mineral with formula . Physical properties Epsomite crystallizes in the orthorhombic system. The normal form is as massive encrustations, while acicular or fibrous crystals are rarely found. It is colorless to white with tints of yellow, green and pink. It is a soft mineral with variable Mohs hardness around 2.0~2.5, and it has a low specific gravity It is readily soluble in water, and absorbs water from the air. It converts to hexahydrate with the loss of one water molecule and a switch to monoclinic structure. The epsomite group includes solid solution series with morenosite (·) and goslarite (·). Etymology It was first systematically described in 1806 for an occurrence near Epsom, Surrey, England, after which it was named. It has been also referred to as "cave cotton" when in its fibrous form. Occurrence Epsomite forms as encrustations or efflorescences on limestone caver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallize
Crystallization is a process that leads to solids with highly organized atoms or molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regular organization. Crystallization can occur by various routes including precipitation from solution, freezing of a liquid, or deposition from a gas. Attributes of the resulting crystal can depend largely on factors such as temperature, air pressure, cooling rate, or solute concentration. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. Crystallizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spring (water)
A spring is a natural exit point at which groundwater emerges from an aquifer and flows across the ground surface as surface water. It is a component of the hydrosphere, as well as a part of the water cycle. Springs have long been important for humans as a source of fresh water, especially in arid regions which have relatively little annual rainfall. Springs are driven out onto the surface by various natural forces, such as gravity and hydrostatic pressure. A spring produced by the emergence of geothermally heated groundwater is known as a hot spring. The yield of spring water varies widely from a volumetric flow rate of nearly zero to more than for the biggest springs. Formation Springs are formed when groundwater flows onto the surface. This typically happens when the water table reaches above the surface level, or if the terrain depresses sharply. Springs may also be formed as a result of karst topography, aquifers or volcanic activity. Springs have also been observe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Mineral
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal Vein (geology), veins and as secondary minerals in the Redox, oxidizing zone of sulfide mineral deposits. The Chromate ion, chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates **Barite BaSO4 **Celestite SrSO4 **Anglesite PbSO4 **Anhydrite CaSO4 **Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O **Chalcanthite CuSO4·5H2O **Kieserite MgSO4·H2O **Starkeyite MgSO4·4H2O **Hexahydrite MgSO4·6H2O **Epsomite MgSO4·7H2O **Meridianiite MgSO4·11H2O **Melanterite FeSO4·7H2O **Antlerite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are not inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain '' water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabolism. ''Photosynthesis'' usually refers to oxygenic photosynthesis, a process that produces oxygen. Photosynthetic organisms store the chemical energy so produced within intracellular organic compounds (compounds containing carbon) like sugars, glycogen, cellulose and starches. To use this stored chemical energy, an organism's cells metabolize the organic compounds through cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth. Some bacteria also perform anoxygenic photosynthesis, which uses bacteriochlorophyll to split hydrogen sulfide as a reductant instead of water, p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy from light. Those pigments are involved in oxygenic photosynthesis, as opposed to bacteriochlorophylls, related molecules found only in bacteria and involved in anoxygenic photosynthesis. Chlorophylls absorb light most strongly in the blue portion of the electromagnetic spectrum as well as the red portion. Conversely, it is a poor absorber of green and near-green portions of the spectrum. Hence chlorophyll-containing tissues appear green because green light, diffusively reflected by structures like cell walls, is less absorbed. Two types of chlorophyll exist in the photosystems of green plants: chlorophyll ''a'' and ''b''. History Chlorophyll was first isolated and named by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plant Nutrition
Plant nutrition is the study of the chemical elements and compounds necessary for plant growth and reproduction, plant metabolism and their external supply. In its absence the plant is unable to complete a normal life cycle, or that the element is part of some essential plant constituent or metabolite. This is in accordance with Justus von Liebig's law of the minimum. The total essential plant nutrients include seventeen different elements: carbon, oxygen and hydrogen which are absorbed from the air, whereas other nutrients including nitrogen are typically obtained from the soil (exceptions include some parasitic or carnivorous plants). Plants must obtain the following mineral nutrients from their growing medium: * The macronutrients: nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), sulfur (S), magnesium (Mg), carbon (C), hydrogen (H), oxygen (O) * The micronutrients (or trace minerals): iron (Fe), boron (B), chlorine (Cl), manganese (Mn), zinc (Zn), copper (Cu), mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bath Salts
Bath salts are water-soluble, pulverized minerals that are added to water to be used for bathing. It is said that these salts improve cleaning, enhance the enjoyment of bathing, and serve as a vehicle for cosmetic agents. Bath salts have been developed that mimic the properties of natural mineral baths or hot springs. Some bath salts contain glycerine so the product will act as an emollient, humectant, or lubricant. Fragrances and colors are often added to bath salts; the fragrances are used to increase the users' enjoyment of the bathing experience. Description Substances often labeled as bath salts include magnesium sulfate (Epsom salts), sodium chloride (table salt), sodium bicarbonate (baking soda), sodium hexametaphosphate (Calgon, amorphous/glassy sodium metaphosphate), sodium sesquicarbonate, sodium citrate and formerly borax. Glycerin, or liquid glycerin, is another common ingredient in bath salts. Fragrances and colors are often added to bath salts; in fact, on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Household Chemical
Household chemicals are non-food chemicals that are commonly found and used in and around the average household. They are a type of consumer goods, designed particularly to assist cleaning, house and yard maintenance, cooking, pest control and general hygiene purposes, often stored in the kitchen or garage. Food additives generally do not fall under this category, unless they have a use other than for human consumption. Additives in general (e.g. stabilizers and coloring found in washing powder and dishwasher detergents) make the classification of household chemicals more complex, especially in terms of health - some of these chemicals are irritants or potent allergens - and ecological effects. Together with non-compostable household waste, the chemicals found in private household commodities pose a serious ecological problem. In addition to having slightly adverse up to seriously toxic effects when swallowed, chemical agents around may contain flammable or corrosive substances ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |