|
Polycatenane
A polycatenane is a chemical substance that, like polymers, is chemically constituted by a large number of units. These units are made up of concatenated rings into a chain-like structure. It consists of mechanically linked catenane structures, via topological Hopf links, resulting in a higher dimensionality than the repeating unit. They are a class of catenanes where the number of macrocycles is greater than two and as catenanes they belong to the big family of mechanically interlocked molecular architectures (MIMAs). The characteristic feature of a polycatenane compound, that distinguishes it from other polymers, is the presence of mechanical bonds in addition to covalent bonds. The rings in this chain-like structure can be separated only when high energy is provided to break at least a covalent bond of the macrocycle. 'n''Catenanes (for large n), which consist solely of the mechanically interlocked cyclic components, can be viewed as “optimized” polycatenanes. The main d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology " mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis. Synthesis There are two primary approaches to the organic synthesis of catenanes. The first is to simply perform a ring-closing reaction with the hope that some of the rings will form around other rings giving the desired catenane product. This so-called "statistical approach" led to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catenane Mobility
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology " mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis. Synthesis There are two primary approaches to the organic synthesis of catenanes. The first is to simply perform a ring-closing reaction with the hope that some of the rings will form around other rings giving the desired catenane product. This so-called "statistical approach" led to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology " mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis. Synthesis There are two primary approaches to the organic synthesis of catenanes. The first is to simply perform a ring-closing reaction with the hope that some of the rings will form around other rings giving the desired catenane product. This so-called "statistical approach" led to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
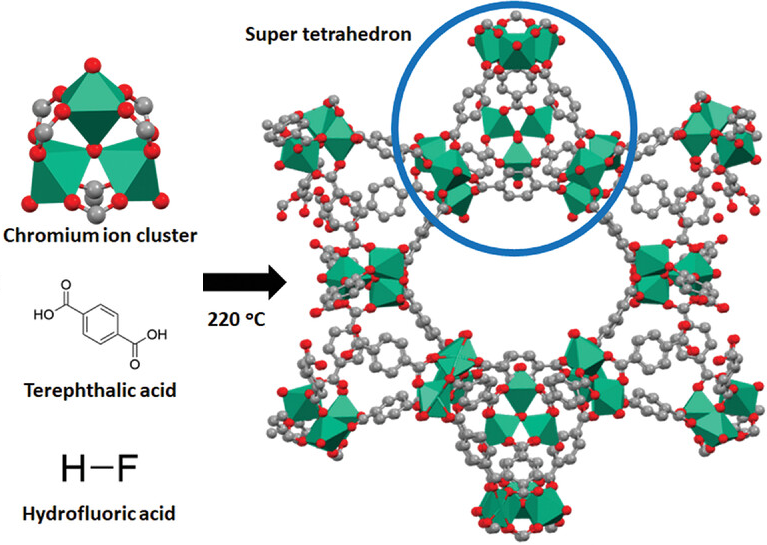
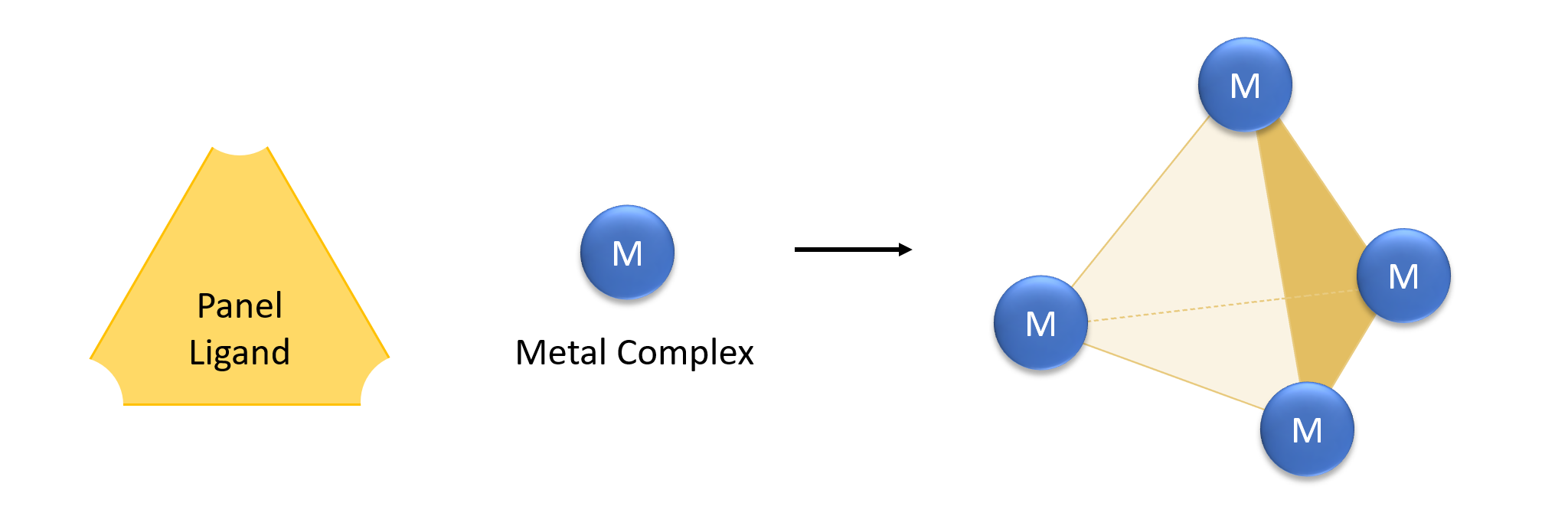
Metal–organic Framework
Metal–organic frameworks (MOFs) are a class of porous polymers consisting of metal cluster compound, clusters (also known as Secondary Building Units - SBUs) coordinated to organic compound, organic ligands to form one-, two- or three-dimensional structures. The organic ligands included are sometimes referred to as "struts" or "linkers", one example being terephthalic acid, 1,4-benzenedicarboxylic acid (BDC). MOFs are classified as reticular materials. More formally, a metal–organic framework is a potentially porous extended structure made from metal ions and organic linkers. An extended structure is a structure whose sub-units occur in a constant ratio and are arranged in a repeating pattern. MOFs are a subclass of coordination networks, which is a coordination compound extending, through repeating coordination entities, in one dimension, but with cross-links between two or more individual chains, loops, or spiro-links, or a coordination compound extending through repeating c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supramolecular Chemistry
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatics, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, coordination complex, metal coordination, hydrophobic effect, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects. Important concepts advanced by supramolecular chemistry include molecular self-assembly, folding (chemistry), molecular folding, molecular recognition, host–gues ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymers
A polymer () is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. Polymers are studied in the fields of polymer science (which includes polymer chemistry and polymer p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal-organic Compound
Metal-organic compounds (jargon: metalorganics, metallo-organics) are a class of chemical compounds that contain metals and organic ligands, but lacking direct metal-carbon bonds. Metal β-diketonates, metal alkoxides, metal dialkylamides, transition metal carboxylate complexes, metal acetylacetonates, and metal phosphine complexes are representative members of this class. Some of metal-organic compounds confer solubility in organic solvents or volatility. Compounds with these properties find applications in materials science for metal organic vapor deposition (MOCVD) or sol-gel processing. Precise definitions of metal-organic compound may vary, however the term may describe: *Organometallic chemistry * Metal coordination complex A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...es ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macromolecule
A macromolecule is a "molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass." Polymers are physical examples of macromolecules. Common macromolecules are biopolymers (nucleic acids, proteins, and carbohydrates). and polyolefins (polyethylene) and polyamides (nylon). Synthetic macromolecules Many macromolecules are synthetic polymers (plastics, synthetic fibers, and synthetic rubber. Polyethylene is produced on a particularly large scale such that ethylene is the primary product in the chemical industry. Macromolecules in nature * Proteins are polymers of amino acids joined by peptide bonds. * DNA and RNA are polymers of nucleotides joined by phosphodiester bonds. These nucleotides consist of a phosphate group, a sugar ( ribose in the case of RNA, deoxyribose in the case of DNA), and a nucleotide base (either adenine, guan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
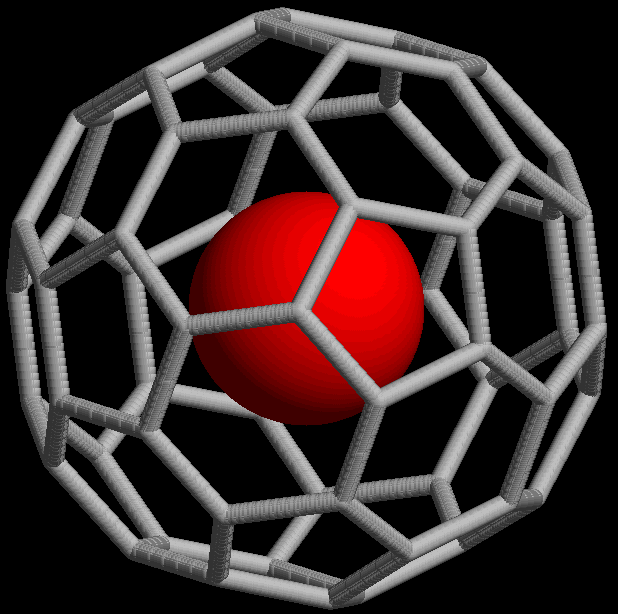
Macromolecular Cages
In host–guest chemistry, macromolecular cages are a type of macromolecule structurally consisting of a three-dimensional chamber surrounded by a molecular framework. Macromolecular cages can be considered large-sized organic molecular cages. Macromolecular cage architectures come in various sizes ranging from 1-50 nm and have varying topologies as well as functions. They can be synthesized through covalent bonding or Molecular self-assembly, self-assembly through non-covalent interactions. Most macromolecular cages that are formed through self-assembly are sensitive to pH, temperature, and solvent polarity. Metal Organic Polyhedra Metal Organic Polyhedra (MOPs) comprise a specific type of self-assembled macromolecular cage that is formed through unique coordination and is typically chemically and thermally stable. MOPs have cage-like frameworks with an enclosed cavity. The discrete self-assembly of metal ions and organic scaffolds to form MOPs into highly symmetrical ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Cage
Coordination cages are three-dimensional ordered structures in solution that act as hosts in host–guest chemistry. They are self-assembled in solution from organometallic precursors, and often rely solely on noncovalent interactions rather than covalent bonds. Coordinate bonds are useful in such supramolecular self-assembly because of their versatile geometries. However, there is controversy over calling coordinate bonds noncovalent, as they are typically strong bonds and have covalent character. The combination of a coordination cage and a guest is a type of inclusion compound. Coordination complexes can be used as "nano-laboratories" for synthesis, and to isolate interesting intermediates. The inclusion complexes of a guest inside a coordination cage show intriguing chemistry as well; often, the properties of the cage will change depending on the guest. Coordination complexes are molecular moieties, so they are distinct from clathrates and metal-organic frameworks. History Chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. Synthesis The formation of macrocycles by ring-closure is called macrocyclization. The central challenge to macrocyclization is that ring-closing reactions do not favor the formation of large rings. Instead, medium sized rings or polymers tend to form. Early macrocyclizations were achieved ketonic decarboxylations for the preparation of terpenoid macrocycles. So, while Ružička was able to produce various macrocycles, the yields were low. This kinetic problem can be addressed by using high-dilution reactions, whereby intramolecular processes are favored relative to polymerizations. Reactions amenable to high dilution include Dieckmann condensation and related based-induced reactions of esters with remote halides. Some macrocyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |