|
Catenane Mobility
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology " mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis. Synthesis There are two primary approaches to the organic synthesis of catenanes. The first is to simply perform a ring-closing reaction with the hope that some of the rings will form around other rings giving the desired catenane product. This so-called "statistical approach" led to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coulomb Force
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that calculates the amount of force between two electrically charged particles at rest. This electric force is conventionally called the ''electrostatic force'' or Coulomb force. Although the law was known earlier, it was first published in 1785 by French physicist Charles-Augustin de Coulomb. Coulomb's law was essential to the development of the theory of electromagnetism and maybe even its starting point, as it allowed meaningful discussions of the amount of electric charge in a particle. The law states that the magnitude, or absolute value, of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them. Coulomb discovered that bodies with like electrical charges repel: Coulomb also showed that oppositely charged bodies attra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
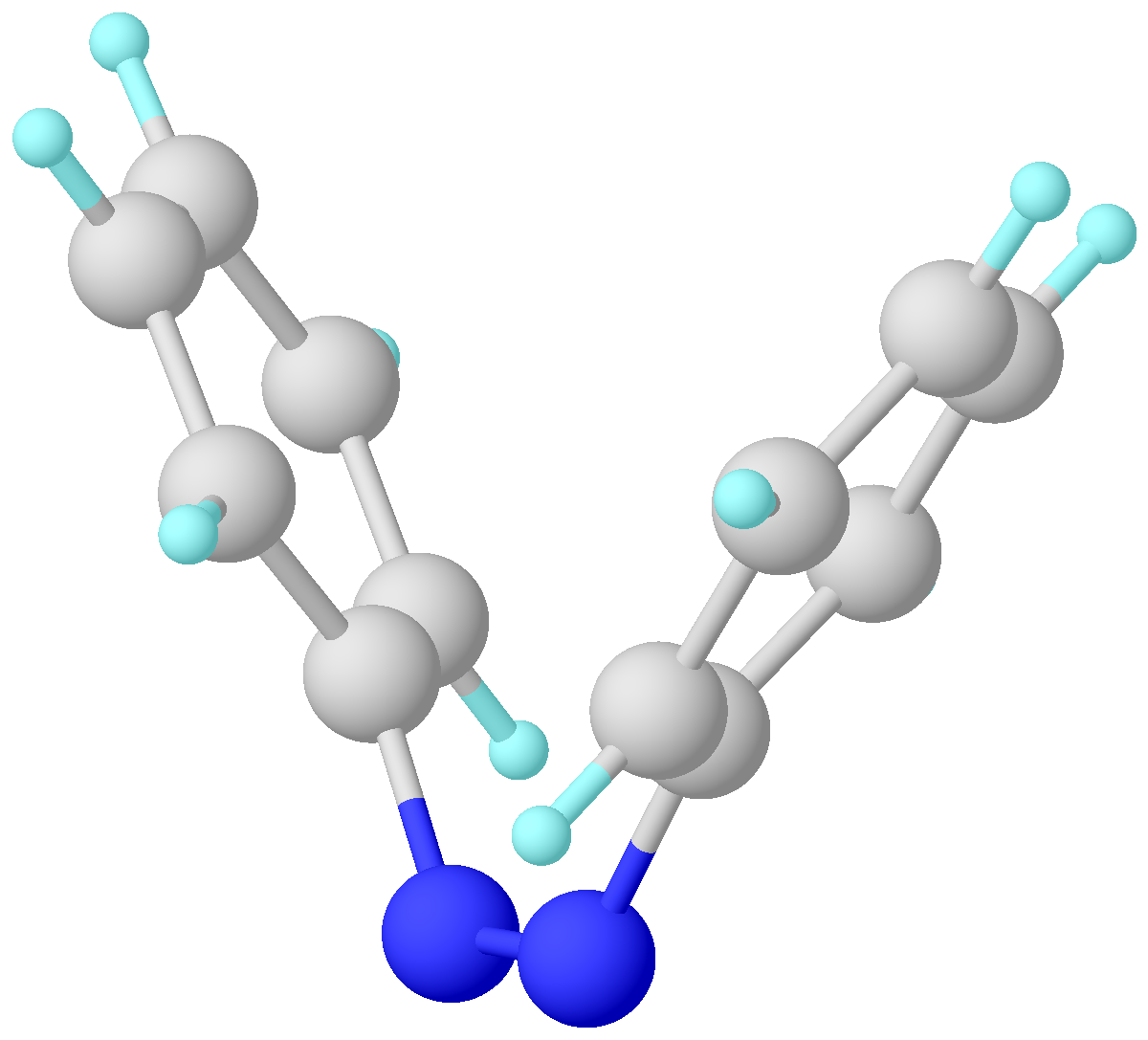
Azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a azo compound, N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar Chemical compound, compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Different classes of azo dyes exist, most notably the ones substituted with heteroaryl rings. Structure and synthesis Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, nitrobenzene is reduced by iron filings in the presence of acetic acid. In the modern synthesis, zinc is the reductant in the presence of a base. Industrial electrosynthesis using nitrobenzene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrathiafulvalene
Tetrathiafulvalene (TTF) is an organosulfur compound with the formula . It is the parent of many tetrathiafulvenes. Studies on these heterocyclic compound contributed to the development of molecular electronics, although no practical applications of TTF emerged. TTF is related to the hydrocarbon fulvalene () by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives. Preparation The high level of interest in TTFs spawned many syntheses of TTF and its analogues. Most preparations entail the coupling of cyclic building blocks such as 1,3-dithiole-2-thion or the related 1,3-dithiole-2-ones. For TTF itself, the synthesis begins with the cyclic trithiocarbonate ( 1,3-dithiole-2-thione), which is ''S''-methylated and then reduced to give (1,3-dithiole-2-yl methyl thioether), which is treated as follows: Protonolysis of a thioether: : Followed by deprotonation of the dithiolium cation with triethylamine: : Redox prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viologen
Viologens are organic compounds with the formula (C5H4NR)2n+. In some viologens, the pyridyl groups are further modified. Viologens are called so, because these compounds produce violet color on reduction [violet + Latin ''gen'', generator of]. The viologen paraquat (R = methyl), is a widely used herbicide. As early as in the 1930s, paraquat was being used as an oxidation-reduction indicator, because it becomes violet on reduction. Other viologens have been commercialized because they can change color reversibly many times through Redox, reduction and oxidation. The name viologen alludes to violet, one color it can exhibit, and the radical ion, radical cation (C5H4NR)2+ is colored intensely blue. Types of viologens As bipyridinium derivatives, the viologens are related to 4,4'-bipyridyl. The basic nitrogen centers in these compounds are alkylated to give viologens: :(C5H4N)2 + 2 RX → [(C5H4NR)2]2+(X−)2 The alkylation is a form of quaternization. When the alkylati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Switch
A molecular switch is a molecule that can be switched between two or more stable or Metastability, metastable states with the use of any external (exogenous) or internal (endogenous) stimuli, such as changes in pH, light, temperature, an electric current, a microenvironment, or in the presence of ions, and other ligands. In some cases, a combination of stimuli is required. Molecular switches are Reversible reaction, reversible. They have been considered for a wide area of possible applications, but the main uses are in photochromic lenses and windows. Biological Stimuli-responsive drug delivery systems, Biological stimuli are endogenous form of stimuli. This involves variation in the physiological changes around the cells, such as variable pH, presence of oxidative or reductive species, and enzymes. In cellular biology, proteins act as intracellular signaling molecules by activating another protein in a signaling pathway. In order to do this, proteins act as molecular switch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantity, physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the thermodynamic efficiency, efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMR Spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic field. This re-orientation occurs with absorption of electromagnetic radiation in the radio frequency region from roughly 4 to 900 MHz, which depends on the isotopic nature of the nucleus and increases proportionally to the strength of the external magnetic field. Notably, the resonance frequency of each NMR-active nucleus depends on its chemical environment. As a result, NMR spectra provide information about individual functional groups present in the sample, as well as about connections between nearby nuclei in the same molecule. As the NMR spectra are unique or highly characteristic to individual compounds and functional groups, NMR spectroscopy is one of the most important methods to identify molecular structures, particularly of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamic Covalent Chemistry
Dynamic covalent chemistry (Commonly abbreviated to DCvC or DCC) is a Chemical synthesis, synthetic strategy employed by chemists to make complex molecular and supramolecular assemblies from discrete molecular building blocks. DCvC has allowed access to complex assemblies such as covalent organic frameworks, molecular knots, polymers, and novel macrocycles. Not to be confused with dynamic combinatorial chemistry, DCvC concerns only covalent bonding interactions. As such, it only encompasses a subset of Supramolecular chemistry, supramolecular chemistries. The underlying idea is that rapid Chemical equilibrium, equilibration allows the coexistence of a variety of different species among which molecules can be selected with desired chemical, pharmaceutical and biological properties. For instance, the addition of a proper template will shift the equilibrium toward the component that forms the complex of higher stability (''thermodynamic template effect''). After the new equilibrium is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamic Combinatorial Chemistry
Dynamic combinatorial chemistry (DCC); also known as constitutional dynamic chemistry (CDC) is a method for the generation of new molecules formed by reversible reaction of simple building blocks under thermodynamic control.Schaufelberger, F.; Timmer, B. J. J.; Ramström, O. Principles of Dynamic Covalent Chemistry. In ''Dynamic Covalent Chemistry: Principles, Reactions, and Applications''; Zhang, W.; Jin, Y., Eds.; John Wiley & Sons: Chichester, 2018; Chapter 1, pp 1–30. The library of these reversibly interconverting building blocks is called a dynamic combinatorial library (DCL).Komáromy, D.; Nowak, P.; Otto, S. Dynamic Combinatorial Libraries. In ''Dynamic Covalent Chemistry: Principles, Reactions, and Applications''; Zhang, W.; Jin, Y., Eds.; John Wiley & Sons: Chichester, 2018; Chapter 2, pp 31–119.Lehn, J.-M.; Ramström, O. Generation and screening of a dynamic combinatorial library. PCT. Int. Appl. WO 20010164605, 2001. All constituents in a DCL are in Thermodynamic equ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sijbren Otto
Sybren Otto (Groningen, 3 August 1971) is Professor of Systems chemistry at the Stratingh Institute for Chemistry, University of Groningen. Career Otto studied chemistry at the University of Groningen and in 1994, he received his Master's degree, focusing on physical organic chemistry and biochemistry, with the distinction cum laude. In 1998, he obtained his PhD, again with the distinction cum laude, from his supervisor Prof. Jan B.F.N. Engberts for his thesis entitled Catalysis of Diels-Alder reactions in water. After his subsequent research in both the United States (in 1998, with Prof. Steven L. Regen) at Lehigh University and in the United Kingdom (first with Prof. Jeremy K.M. Sanders and then, from 2001 onwards, as a Royal Society University Research Fellow, both at the University of Cambridge), he was appointed assistant professor at the University of Groningen in 2009. In 2011, he was promoted to associate professor and in 2016, to full professor. From 2014 to 2019, he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jeremy Sanders
Jeremy Keith Morris Sanders (born 3 May 1948) is a British chemist and Emeritus Professor in the Department of Chemistry at the University of Cambridge. He is also Editor-in-Chief of Royal Society Open Science. He is known for his contributions to many fields including NMR spectroscopy and supramolecular chemistry. He served as the Pro-Vice-Chancellor for Institutional Affairs at the University of Cambridge, 2011–2015. Education Educated in London at Southmead Primary School and Wandsworth Comprehensive School, he then studied chemistry at Imperial College London where he graduated with a Bachelor of Science degree in 1969 and was awarded the Edmund White Prize. During 1969–72 he carried out his PhD research on lanthanide shift reagents, especially Eu(DPM), the original reagent developed before Eu(FOD) at Churchill College, Cambridge, supervised by Dudley Williams. Career and Research Elected a fellow of Christ's College, Cambridge, in 1972, he spent a postdoctoral y ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |