azobenzene on:
[Wikipedia]
[Google]
[Amazon]
Azobenzene is a photoswitchable
 Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method,
Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, 
chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
compound composed of two phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
rings linked by a N=N double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
. It is the simplest example of an aryl azo compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups).
IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted ...
. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes
Juan de Guillebon, better known by his stage name DyE, is a French musician. He is known for the music video of the single "Fantasy (DyE song), Fantasy" from his first album ''Taki 183 (album), Taki 183''. This video became popular, attracting ...
. Different classes of azo dyes exist, most notably the ones substituted with heteroaryl rings.
Structure and synthesis
 Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method,
Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, nitrobenzene
Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced ...
is reduced by iron filings in the presence of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
. In the modern synthesis, zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
is the reductant in the presence of a base. Industrial electrosynthesis using nitrobenzene is also employed.
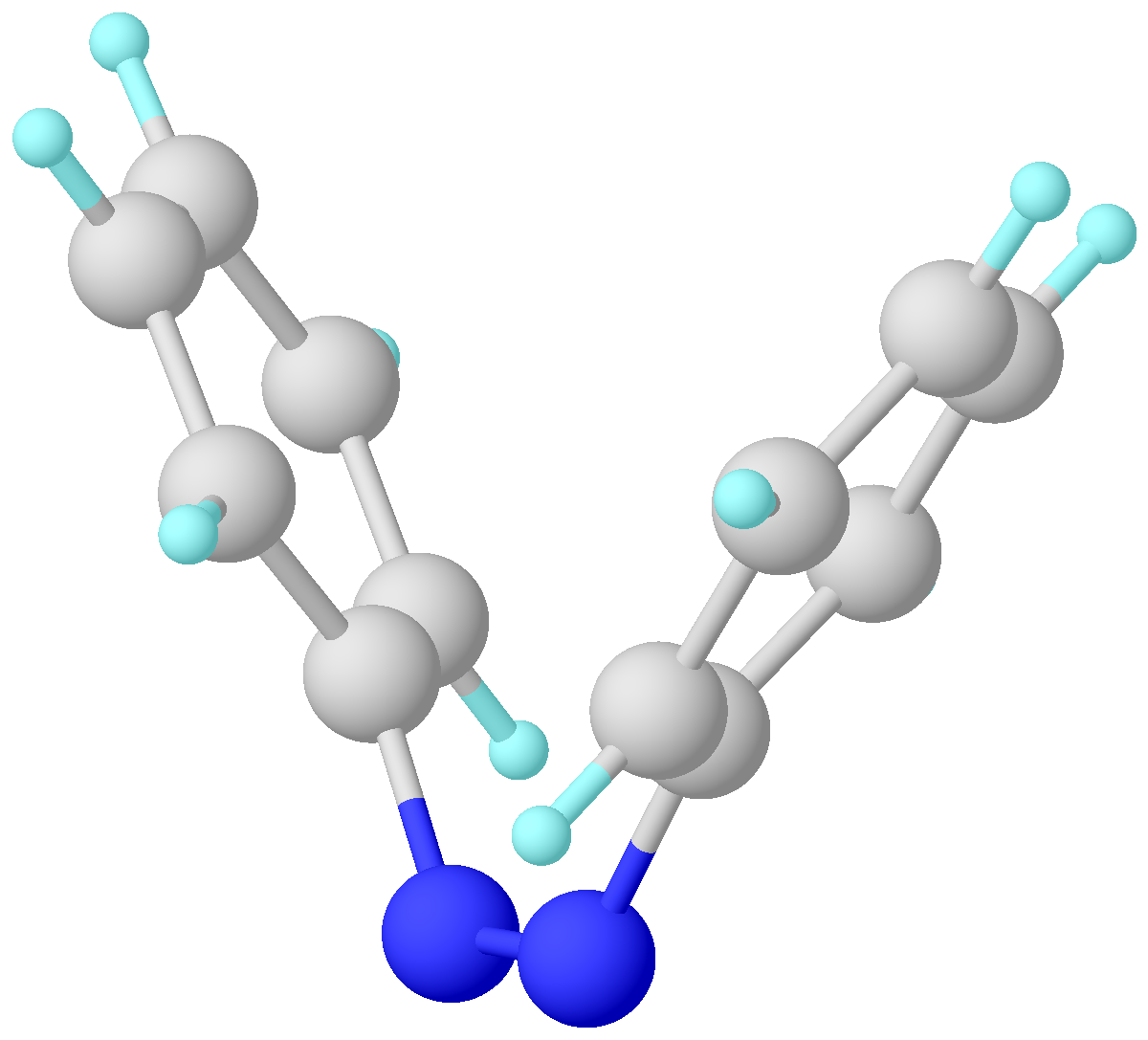
''trans''-Azobenzene isomer is planar with an N-N distance of 1.189 Å. ''cis''-Azobenzene is nonplanar with a C-N=N-C dihedral angle of 173.5° and an N-N distance of 1.251 Å. The trans isomer is more stable by approximately 50 kJ/mol, and the barrier to isomerization in the ground state is approximately 100 kJ/mol.

Reactions
Azobenzene is a weak base, but undergoes protonation at one nitrogen with a pKa = -2.95. It functions as aLewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
, e.g. toward boron trihalides. It binds to low valence metal centers, e.g. Ni(Ph2N2)(PPh3)2 is well characterized.
Azobenzene oxidizes to give azoxybenzene. Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
gives diphenylhydrazine.
Trans–cis isomerization
Azobenzene (and derivatives) undergo photoisomerization oftrans
Trans- is a Latin prefix meaning "across", "beyond", or "on the other side of".
Used alone, trans may refer to:
Sociology
* Trans, a sociological term which may refer to:
** Transgender, people who identify themselves with a gender that di ...
and cis isomers. cis-Azobenzene relaxes back, in dark, to the trans isomer. Such thermal relaxation is slow at room temperature. The two isomers can be switched with particular wavelengths of light: ultraviolet light, which corresponds to the energy gap of the π-π* (''S2'' state) transition, for trans-to-cis conversion, and blue light, which is equivalent to that of the n-π* (''S1'' state) transition, for cis-to-trans isomerization. For a variety of reasons, the ''cis'' isomer is less stable than the trans (for instance, it has a distorted configuration and is less delocalized than the trans configuration). Photoisomerization allows for reversible energy storage (as photoswitches).
Spectroscopic classification
The wavelengths at which azobenzene isomerization occurs depends on the particular structure of each azo molecule, but they are typically grouped into three classes: the azobenzene-type molecules, the aminoazobenzenes, and the pseudo- stilbenes. These azos are yellow, orange, and red, respectively, owing to the subtle differences in their electronic absorption spectra. The compounds similar to the unsubstituted azobenzene exhibit a low-intensity n-π* absorption in the visible region, and a much higher intensity π-π* absorption in theultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
. Azos that are ortho- or para-substituted with electron-donating groups (such as amino
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s), are classified as aminoazobenzenes, and tend to closely spaced n-π* and π-π* bands in the visible. The pseudo-stilbene class is characterized by substituting the 4 and 4' positions of the two azo rings with electron-donating and electron-withdrawing groups (that is, the two opposite ends of the aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
system are functionalized). The addition of this push-pull configuration results in a strongly asymmetric electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
distribution, which modifies a host of optical properties. In particular, it shifts the absorption spectra of the ''trans'' and the ''cis'' isomers, so that they effectively overlap. Thus, for these compounds a single wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
of light in the visible region will induce both the forward and reverse isomerization. Under illumination, these molecules cycle between the two isomeric states.
Photophysics of isomerization
The photo-isomerization of azobenzene is extremely rapid, occurring on picosecond timescales. The rate of the thermal back-relaxation varies greatly depending on the compound: usually hours for azobenzene-type molecules, minutes for aminoazobenzenes, and seconds for the pseudo-stilbenes. The mechanism of isomerization has been the subject of some debate, with two pathways identified as viable: a ''rotation'' about the N-N bond, with disruption of the double bond, or via an ''inversion'', with a semi-linear and hybridized transition state. It has been suggested that the ''trans''-to-''cis'' conversion occurs via rotation into the ''S2'' state, whereas inversion gives rise to the ''cis''-to-''trans'' conversion. It is still under discussion which excited state plays a direct role in the series of the photoisomerization behavior. However, the latest research utilizing femtosecond transient absorption spectroscopy has suggested that the ''S2'' state undergoes internal conversion to the ''S1'' state, and then the ''trans''-to-''cis'' isomerization proceeds. Recently another isomerization pathway has been proposed by Diau, the "concerted inversion" pathway in which both CNN bond angles bend at the same time. There is experimental and computational evidence for the existence of a multistate rotation mechanism involving a triplet state.Photoinduced motions
The photo-isomerization of azobenzene is a form of light-induced molecular motion. This isomerization can also lead to motion on larger length scales. For instance, polarized light will cause the molecules to isomerize and relax in random positions. However, those relaxed (''trans'') molecules that fall perpendicular to the incoming light polarization will no longer be able to absorb, and will remain fixed. Thus, there is a statistical enrichment of chromophores perpendicular to polarized light (orientational hole burning). Polarized irradiation will make an azo-materialanisotropic
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit ver ...
and therefore optically birefringent
Birefringence, also called double refraction, is the optical property of a material having a refractive index that depends on the polarization and propagation direction of light. These optically anisotropic materials are described as birefring ...
and dichroic
In optics, a dichroic material is either one which causes visible light to be split up into distinct beams of different wavelengths (colours) (not to be confused with dispersion), or one in which light rays having different polarizations are ab ...
. This photo-orientation can also be used to orient other materials (especially in liquid crystal
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as i ...
systems).
Miscellaneous
Azobenzene undergoes ortho-metalation by metal complexes, e.g. dicobalt octacarbonyl: Info about the carcinogenicity of Azobenzene can be found on the EPA site, where it has been classified as a "probable human carcinogen" based on evidence of carcinogenicity in animals.References
Cited sources
*Further reading
*Of historic interest: * * * * * * *{{ cite journal , author1=Banghart, M. R. , author2=Volgraf, M. , author3=Trauner, D. , title = Engineering light-gated ion channels , journal = Biochemistry , volume = 45 , issue = 51 , pages = 15129–15141 , date=December 2006 , pmid = 17176035 , doi = 10.1021/bi0618058 , citeseerx=10.1.1.70.6273 Phenyl compounds Azo compounds