|
PHGDH
Phosphoglycerate dehydrogenase (PHGDH) is an enzyme that catalyzes the chemical reactions :3-phospho-D-glycerate + NAD+ \rightleftharpoons 3-phosphonooxypyruvate + NADH + H+ :2-hydroxyglutarate + NAD+ \rightleftharpoons 2-oxoglutarate + NADH + H+ The two substrates of this enzyme are 3-phospho-D-glycerate and NAD+, whereas its 3 products are 3-phosphohydroxypyruvate, NADH, and H+ It is also possible that two substrates of this enzyme are 2-hydroxyglutarate and NAD+, whereas its 3 products are 2-oxoglutarate, NADH, and H+. As of 2012, the most widely studied variants of PHGDH are from the ''E. coli'' and ''M. tuberculosis'' genomes. In humans, this enzyme is encoded by the ''PHGDH'' gene. Function 3-Phosphoglycerate dehydrogenase catalyzes the transition of 3-phosphoglycerate into 3-phosphohydroxypyruvate, which is the committed step in the phosphorylated pathway of L-serine biosynthesis. It is also essential in cysteine and glycine synthesis, which lie further downst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
D-glycerate Dehydrogenase Deficiency
D-glycerate dehydrogenase deficiency (or 3-phosphoglycerate dehydrogenase deficiency, PHGDH deficiency, PHGDHD) is a rare autosomal metabolic disease where the young patient is unable to produce an enzyme necessary to convert 3-phosphoglycerate into 3-phosphohydroxypyruvate, which is the only way for humans to synthesize serine. This disorder is called Neu–Laxova syndrome in neonates. Symptoms and signs Cause Homozygous or compound heterozygous mutations in 3-phosphoglycerate dehydrogenase (PHGDH) cause Neu-Laxova syndrome and phosphoglycerate dehydrogenase deficiency. Mechanism 3-Phosphoglycerate dehydrogenase catalyzes the transition of 3-phosphoglycerate into 3-phosphohydroxypyruvate, which is the committed step in the phosphorylated pathway of L-serine biosynthesis. It is also essential in cysteine and glycine Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neu–Laxova Syndrome
Neu–Laxova syndrome (NLS, also known as Neu syndrome; Neu-Povýsilová syndrome; or 3-phosphoglycerate dehydrogenase deficiency, neonate form) is a rare autosomal recessive disorder characterized by severe intrauterine growth restriction and multiple congenital malformations. Neu–Laxova syndrome is a very severe disorder, leading to stillbirth or death shortly after birth. It was first described by Dr. Richard Neu in 1971 and Dr. Renata Laxova in 1972 as a lethal disorder in siblings with multiple malformations. Neu–Laxova syndrome is an extremely rare disorder with fewer than 100 cases reported in medical literature. Signs and symptoms Neu-Laxova syndrome presents with severe malformations leading to prenatal or neonatal death. Typically, NLS involves characteristic facial features, decreased fetal movements and skin abnormalities. Fetuses or newborns with Neu–Laxova syndrome have typical facial characteristics which include proptosis (bulging eyes) with eyelid malform ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-phospho-D-glycerate
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. The anion is often termed as PGA when referring to the Calvin-Benson cycle. In the Calvin-Benson cycle, 3-phosphoglycerate is typically the product of the spontaneous scission of an unstable 6-carbon intermediate formed upon CO2 fixation. Thus, two equivalents of 3-phosphoglycerate are produced for each molecule of CO2 that is fixed. In glycolysis, 3-phosphoglycerate is an intermediate following the dephosphorylation ( reduction) of 1,3-bisphosphoglycerate. Glycolysis In the glycolytic pathway, 1,3-bisphosphoglycerate is dephosphorylated to form 3-phosphoglyceric acid in a coupled reaction producing two ATP via substrate-level phosphorylation. The single phosphate group left on the 3-PGA molecule then moves from an end carbon to a centra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all chemical compound, compounds containing covalent bond, covalently bound H atoms. In this broad and potentially archaic sense, water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. In covalent compounds, it implies hydrogen is attached to a less electronegative chemical element, element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form Binary compounds of hydrogen, binary compounds with hydrogen, the exceptions being helium, He, neon, Ne, argon, Ar, krypton, Kr, promethium, Pm, osmium, Os, iridium, Ir, radon, Rn, francium, Fr, and radium, Ra. exotic atom#exotic molecules, Exotic molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Compound Heterozygosity
In medical genetics, compound heterozygosity is the condition of having two or more heterogeneous recessive alleles at a particular locus that can cause genetic disease in a heterozygous state; that is, an organism is a compound heterozygote when it has two recessive alleles for the same gene, but with those two alleles being different from each other (for example, both alleles might be mutated but at different locations). Compound heterozygosity reflects the diversity of the mutation base for many autosomal recessive genetic disorders; mutations in most disease-causing genes have arisen many times. This means that many cases of disease arise in individuals who have two unrelated alleles, who technically are heterozygotes, but both the alleles are defective. These disorders are often best known in some classic form, such as the homozygous recessive case of a particular mutation that is widespread in some population. In its compound heterozygous forms, the disease may have lower pene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group (which is in the protonated form when the lysine is dissolved in water at physiological pH), an α-carboxylic acid group (which is in the deprotonated form when the lysine is dissolved in water at physiological pH), and a side chain (which is partially protonated when the lysine is dissolved in water at physiological pH), and so it is classified as a basic, charged (in water at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an Amine, α-amino group (which is in the protonated –NH3+ form under Physiological condition, biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential amino acid, essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is Genetic code, encoded by the Genetic code, codons CAU and CAC. Histidine was first isolated by Albrecht Kossel and Sven Gustaf Hedin in 1896. The name stems from its discovery in tissue, from ''histós'' "tissue". It is also a Precursor (chemistry), precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical (chemistry), radical is histidyl. Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
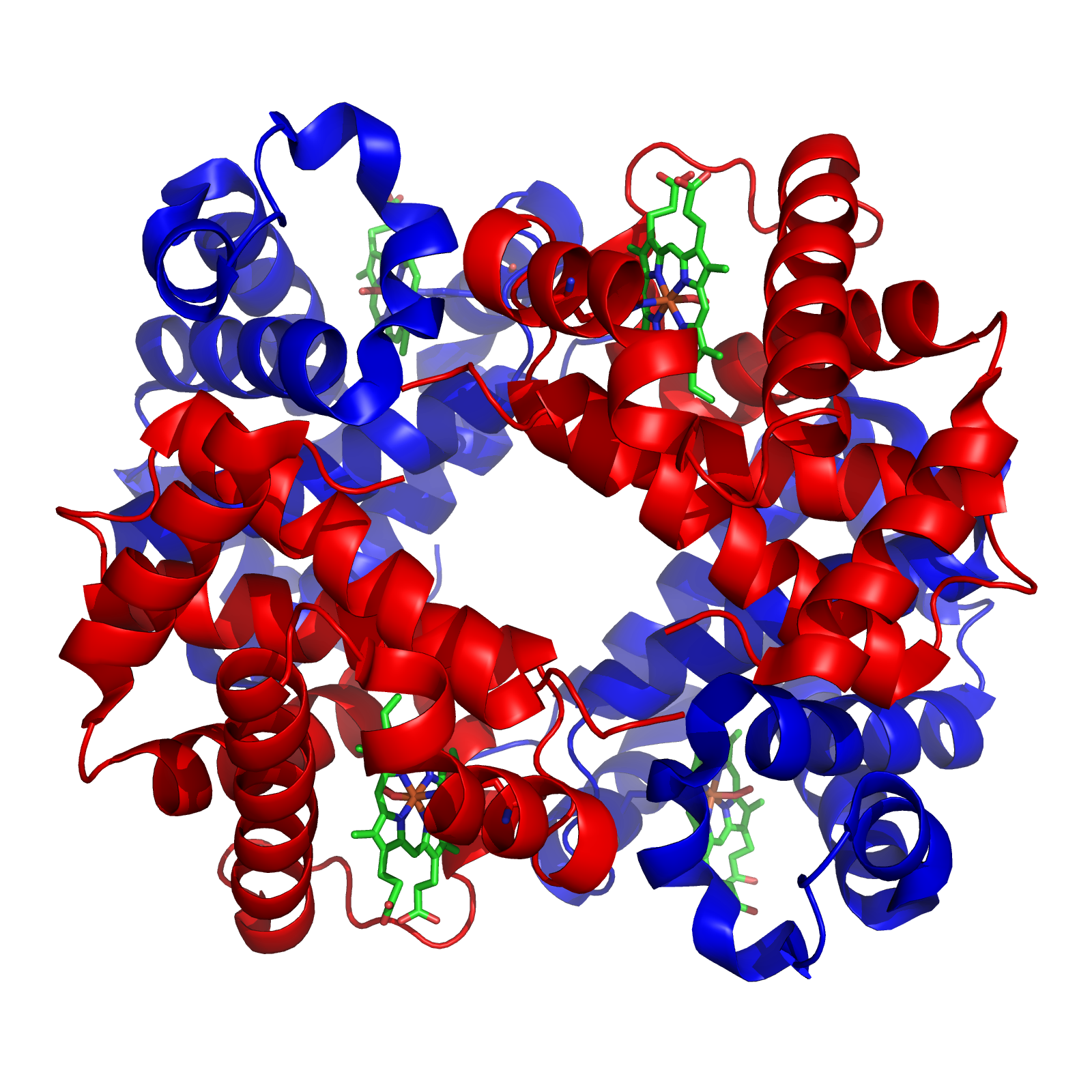
Tetramer
A tetramer () (''tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula Ti(OCH3)4, which is tetrameric in solid state and has the molecular formula Ti4(OCH3)16. An example from organic chemistry is kobophenol A, a substance that is formed by combining four molecules of resveratrol. In biochemistry, it similarly refers to a biomolecule formed of four units, that are the same ( homotetramer), i.e. as in Concanavalin A or different ( heterotetramer), i.e. as in hemoglobin. Hemoglobin has 4 similar sub-units while immunoglobulins have 2 very different sub-units. The different sub-units may have each their own activity, such as binding biotin in avidin tetramers, or have a common biological property, such as the allosteric binding of oxygen Oxygen is a chemical element; it has chemical symbol, symbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cooperativity
Cooperativity is a phenomenon displayed by systems involving identical or near-identical elements, which act dependently of each other, relative to a hypothetical standard non-interacting system in which the individual elements are acting independently. One manifestation of this is enzymes or receptors that have multiple binding sites where the affinity of the binding sites for a ligand is ''apparently'' increased, positive cooperativity, or decreased, negative cooperativity, upon the binding of a ligand to a binding site. For example, when an oxygen atom binds to one of hemoglobin's four binding sites, the affinity to oxygen of the three remaining available binding sites increases; i.e. oxygen is more likely to bind to a hemoglobin bound to one oxygen than to an unbound hemoglobin. This is referred to as cooperative binding. We also see cooperativity in large chain molecules made of many identical (or nearly identical) subunits (such as DNA, proteins, and phospholipids), when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and NADH, reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes. The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, can occur in the Great Oxygenation Event, oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal ions, meaning this is a plausible prebiotic pathway for abiogenesis. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Kar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |