|
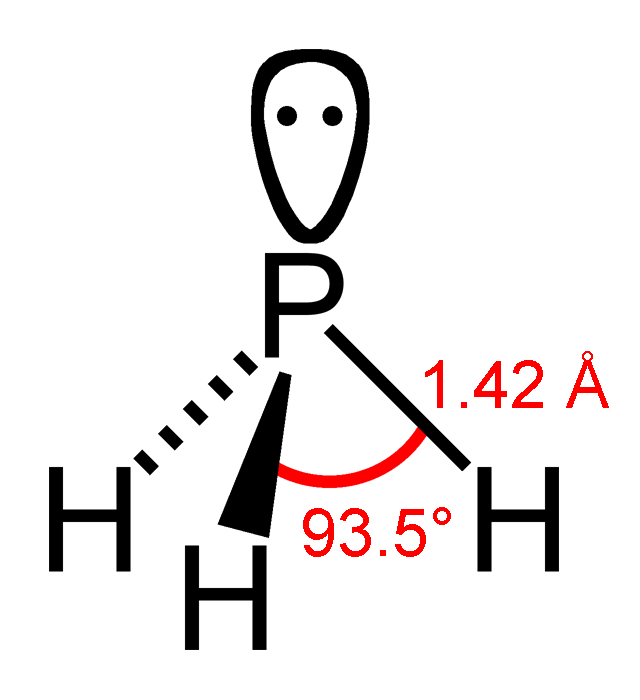
NH3
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula . A stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, refrigerants, explosives, cleaning agents, and is a precursor for numeous chemicals. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals. In many countries, it is classified as an extremely hazardous substance. Ammonia is toxic, causing damage to cells and tissues. For this reason it is excreted by most animals in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the cellular metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. ''Urea'' is Neo-Latin, , , itself from Proto-Indo-European ''*h₂worsom''. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor base (chemistry), alkaline. The body uses it in many processes, most notably metabolic waste#Nitrogen wastes, nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleus) to ammonia (). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by Organic compound, organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it. Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted–Lowry acid–base theory, Brønsted acids (proton donors): : The ammonium ion is mildly acidic, reacting with Brønsted bases to return ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing Polymeric foam, polymer foams, but applications also include its uses as a precursor (chemistry), precursor to pharmaceuticals and agrochemicals, as well as a long-term storable propellant for in-outer space, space spacecraft propulsion. Additionally, hydrazine is used in various rocket propellant, rocket fuels and to prepare the gas precursors used in airbags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. , approximately 120,000 tons of hydrazine hydrate (corresponding to a 64% solution of hydrazine in water by weight) we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia (data Page)
This page provides supplementary chemical data on ammonia. Structure and properties Thermodynamic properties Vapor–liquid equilibrium data Table data (above) obtained from ''CRC Handbook of Chemistry and Physics'' 44th ed. The (s) notation indicates equilibrium temperature of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid. Vapor-pressure formula for ammonia:Lange's Handbook of Chemistry, 10th ed. page 1436. : log10''P'' = ''A'' – ''B'' / (''T'' − ''C''), where ''P'' is pressure in k Pa, and ''T'' is temperature in kelvins; : ''A'' = 6.67956, ''B'' = 1002.711, ''C'' = 25.215 for ''T'' = 190 K through 333 K. Heat capacity of liquid and vapor --> obtained from CHERIC. , - , , - Spectral data Regulatory data Safety data sheet The handling of this chemical may incur notable safety precautions... It is highly recommend that you seek the Safety Data Sheet ( safety data sheet, SDS) for this chemical from a reliable source an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fertiliser
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrition, plant nutrients. Fertilizers may be distinct from Liming (soil), liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and Agrochemical, industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment, or hand-tool methods. Historically, fertilization came from natural or organic sources: compost, Manure, animal manure, Human waste, human manure, harvested minerals, crop rotations, and byproducts of human-nature industries (e.g. Fish meal, fish processing waste, or B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pnictogen Hydride
Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen ( or ; from "to choke" and -gen, "generator") atoms (elements of group 15: nitrogen, phosphorus, arsenic, antimony, bismuth, and moscovium) covalently bonded to hydrogen. Pnictogen trihydrides The simplest series has the chemical formula XH3 (less commonly H3X), with X representing any of the pnictogens. They take on the pyramidal structure (as opposed to the trigonal planar arrangement of the group 13 hydrides), and therefore are polar. These pnictogen trihydrides are generally increasingly unstable and poisonous with heavier elements. Some properties of the pnictogen trihydrides follow: These gases have no smell in pure form, instead gaining it when in contact with air. Ammonia has an infamous, intense odour resembling urine and/or fish, commonly the result of the decomposition of urea. Phosphine smells like fish or garlic, and stibine like rotten eggs, similar to hydrogen sulfide a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Hydroxide
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although the name ammonium hydroxide suggests a Salt (chemistry), salt with the chemical formula, composition , it is impossible to isolate samples of NH4OH. The ions and OH− do not account for a significant fraction of the total amount of ammonia except in extremely dilute solutions. The concentration of such solutions is measured in units of the Baumé scale (density), with 26 degrees Baumé (about 30% of ammonia by weight at ) being the typical high-concentration commercial product. Basicity of ammonia in water In aqueous solution, ammonia deprotonation, deprotonates a small fraction of the water to give ammonium and hydroxide according to the following chemical equilibrium, equilibrium: : NH3 + H2O ⇌ + OH−. In a 1 Molar concentra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diammonium Phosphate
Diammonium phosphate (DAP; IUPAC name diammonium hydrogen phosphate; chemical formula (NH4)2(HPO4)) is one of a series of water- soluble ammonium phosphate salts that can be produced when ammonia reacts with phosphoric acid. Solid diammonium phosphate shows a dissociation pressure of ammonia as given by the following expression and equation: : At 100 °C, the dissociation pressure of diammonium phosphate is approximately 5 mmHg. According to the diammonium phosphate MSDS from CF Industries, Inc., decomposition starts as low as 70 °C: "Hazardous Decomposition Products: Gradually loses ammonia when exposed to air at room temperature. Decomposes to ammonia and monoammonium phosphate at around 70 °C (158 °F). At 155 °C (311 °F), DAP emits phosphorus oxides, nitrogen oxides and ammonia." Uses DAP is used as a fertilizer. When applied as plant fertilizer, it temporarily increases the soil pH, but over a long term the treated ground becomes more acidic than before, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azanide
Azanide is the IUPAC-sanctioned name for the anion . The term is obscure; derivatives of are almost invariably referred to as amides, despite the fact that amide also refers to the Organic chemistry, organic functional group –. The anion is the conjugate base of ammonia, so it is formed by Ammonia#Self-dissociation, the self-ionization of ammonia. It is produced by deprotonation of ammonia, usually with strong bases or an alkali metal. Azanide has a H–N–H bond angle of 104.5°. Alkali metal derivatives The alkali metal derivatives are best known, although usually referred to as alkali metal amides. Examples include lithium amide, sodium amide, and potassium amide. These salt-like solids are produced by treating liquid ammonia with strong bases or directly with the alkali metals (blue liquid ammonia solutions due to the solvated electron): :, where M = Li, Na, K Silver(I) amide () is prepared similarly. Transition metal complexes of the amido ligand are often produced by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully. Solids Many salts and solids can be dried using heat, or under vacuum. Desiccators can also be used to store reagents in dry conditions. Common desiccants include phosphorus pentoxide and silica gel. Chemists may also require dry glassware for sensitive reactions. This can be achieved by drying glassware in an oven, by flame, or under vacuum. Dry solids can be produced by freeze-drying, which is also known as lyophilization. Liquids or solvents In many cases, the presence of water can prevent a reaction from happening, or cause undesirable products to form. To prevent this, anhydrous solvents must be used when perform ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |