|
N-Propyl Bromide
1-Bromopropane (also known as ''n''-propyl bromide or nPB) is a bromoalkane with the chemical formula CH3CH2CH2Br. It is a colorless, flammable liquid that is used as a solvent. It has a characteristic hydrocarbon odor. Its industrial applications increased dramatically in the 21st century due to the phasing out of chlorofluorocarbons and chloroalkanes such as 1,1,1-trichloroethane under the Montreal Protocol. It was also used as a dry cleaning solvent as a substitute for perchloroethylene for a short time in the United States. 1-Bromopropane is highly neurotoxic and possibly carcinogenic to humans. Preparation Industrial routes to 1-bromopropane involve free-radical additions to the corresponding alkenes. In this way, the anti-Markovnikov product is obtained.David Ioffe, Arieh Kampf "Bromine, Organic Compounds" in Kirk-Othmer Encyclopedia of Chemical Technology 2002 by John Wiley & Sons. . Alternatively, ''n''propanol may be substitutively brominated. The latter reaction i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoethane
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr (which is also used as an abbreviation for ethidium bromide). This volatile compound has an ether-like odor. Preparation The preparation of EtBr stands as a model for the synthesis of bromoalkanes in general. It is usually prepared by the addition of hydrogen bromide to ethene: :H2C=CH2 + HBr → H3C-CH2Br Bromoethane is inexpensive and would rarely be prepared in the laboratory. A laboratory synthesis includes reacting ethanol with a mixture of hydrobromic and sulfuric acids. An alternate route involves refluxing ethanol with phosphorus and bromine; phosphorus tribromide is generated ''in situ''. Uses In organic synthesis, EtBr is the synthetic equivalent of the ethyl carbocation (Et+) synthon. In reality, such a cation is not actually formed. For example, carboxylates salts are converted to ethyl esters, carbanions to ethylated derivatives, thioure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Tribromide
Phosphorus tribromide is a colourless liquid with the formula P Br3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the laboratory for the conversion of alcohols to alkyl bromides. Preparation PBr3 is prepared by treating red phosphorus with bromine. An excess of phosphorus is used in order to prevent formation of PBr5: :P4 + 6 Br2 → 4 PBr3 Because the reaction is highly exothermic, it is often conducted in the presence of a diluent such as PBr3. Phosphorus tribromide is also generated in situ from red phosphorus and bromine. Reactions Phosphorus tribromide, like PCl3 and PF3, has both properties of a Lewis base and a Lewis acid. For example, with a Lewis acid such as boron tribromide it forms stable 1 :1 adducts such as Br3B · PBr3. At the same time PBr3 can react as an electrophile or Lewis acid in many of its reactions, for example with amines. An important reaction of PBr3 is with alcohols, where it replaces an OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
American Conference Of Governmental Industrial Hygienists
The American Conference of Governmental Industrial Hygienists (ACGIH) is a professional association of industrial hygienists and practitioners of related professions, with headquarters in Cincinnati, Ohio. One of its goals is to advance worker protection by providing timely, objective, scientific information to occupational and environmental health professionals. History The National Conference of Governmental Industrial Hygienists (NCGIH) convened on June 27, 1938, in Washington, D.C. NCGIH's original constitution limited full membership to two representatives from each governmental industrial hygiene agency. Associate membership was made available to other professional personnel of the agencies holding full memberships, and also to personnel of educational institutions engaged in teaching industrial hygiene. Governmental industrial hygiene personnel of other countries were eligible for affiliated membership. The Conference came into being with 59 members, one affiliated me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxic Substances Control Act Of 1976
The Toxic Substances Control Act (TSCA) is a United States law, passed by the United States Congress, Congress in 1976 and administered by the United States United States Environmental Protection Agency, Environmental Protection Agency (EPA), that regulates chemicals not regulated by other U.S. federal statutes, including chemicals already in commerce and the introduction of new chemicals.Auer, Charles, Frank Kover, James Aidala, Marks Greenwood“Toxic Substances: A Half Century of Progress.”EPA Alumni Association. March 2016. When the TSCA was put into place, all existing chemicals were considered to be safe for use and subsequently Grandfather Clause, grandfathered in. Its three main objectives are to assess and regulate new commercial chemicals before they enter the market, to regulate chemicals already existing in 1976 that posed an "unreasonable risk of injury to health or the environment", as for example Polychlorinated biphenyl, PCBs, lead, mercury and radon, and to re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
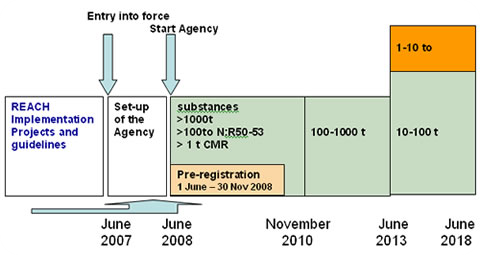
Registration, Evaluation, Authorisation And Restriction Of Chemicals
Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) is a European Union regulation dating from 18 December 2006, amended on 16 December 2008 by Regulation (EC) No 1272/2008. REACH addresses the production and use of chemical substances, and their potential impacts on both human health and the environment. Its 849 pages took seven years to pass, and it has been described as the most complex legislation in the Union's history and the most important in 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world. REACH entered into force on 1 June 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH. Overview When REACH is fully in force, it will require all companies manufacturing or importing chemical substances into the European Union in quantities of o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Cleaning
Dry cleaning is any cleaning process for clothing and textiles using a solvent other than water. Clothes are instead soaked in a water-free liquid solvent (usually non-polar, as opposed to water which is a Solvent#Solvent classifications, polar solvent). Perchloroethylene (known in the industry as "perc") is the most commonly used solvent, although alternative solvents such as hydrocarbons, and decamethylcyclopentasiloxane are also used. Most natural fibers can be washed in water but some synthetics (e.g., viscose) react poorly with water and should be dry cleaned if possible. If not, this could result in changes in texture, strength, and shape. Additionally, certain specialty fabrics, including silk and rayon, may also benefit from dry cleaning to prevent damage. History The ancient Greeks and Romans had some waterless methods to clean textiles, involving the use of powdered chemicals and absorbent clay (fuller's earth). By the 1700s, the French were using turpentine-based s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perchloroethylene
Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc (or PERC), and PCE, is a chlorocarbon with the formula . It is a non-flammable, stable, colorless and heavy liquid widely used for dry cleaning of fabrics and occasionally as a highly effective automotive brake cleaner. It has a mildly sweet, sharp odor, detectable by most people at a concentration of 50 ppm. Tetrachloroethylene is regarded as a toxic substance, a human health hazard, and an environmental hazard. In 2020, the United States Environmental Protection Agency stated that "tetrachloroethylene exposure may harm the nervous system, liver, kidneys, and reproductive system, and may be harmful to unborn children", and reported that numerous toxicology agencies regard it as a carcinogen. History and production French chemist Henri Victor Regnault first synthesized tetrachloroethylene in 1839 by thermal decomposition of hexachloroethane fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
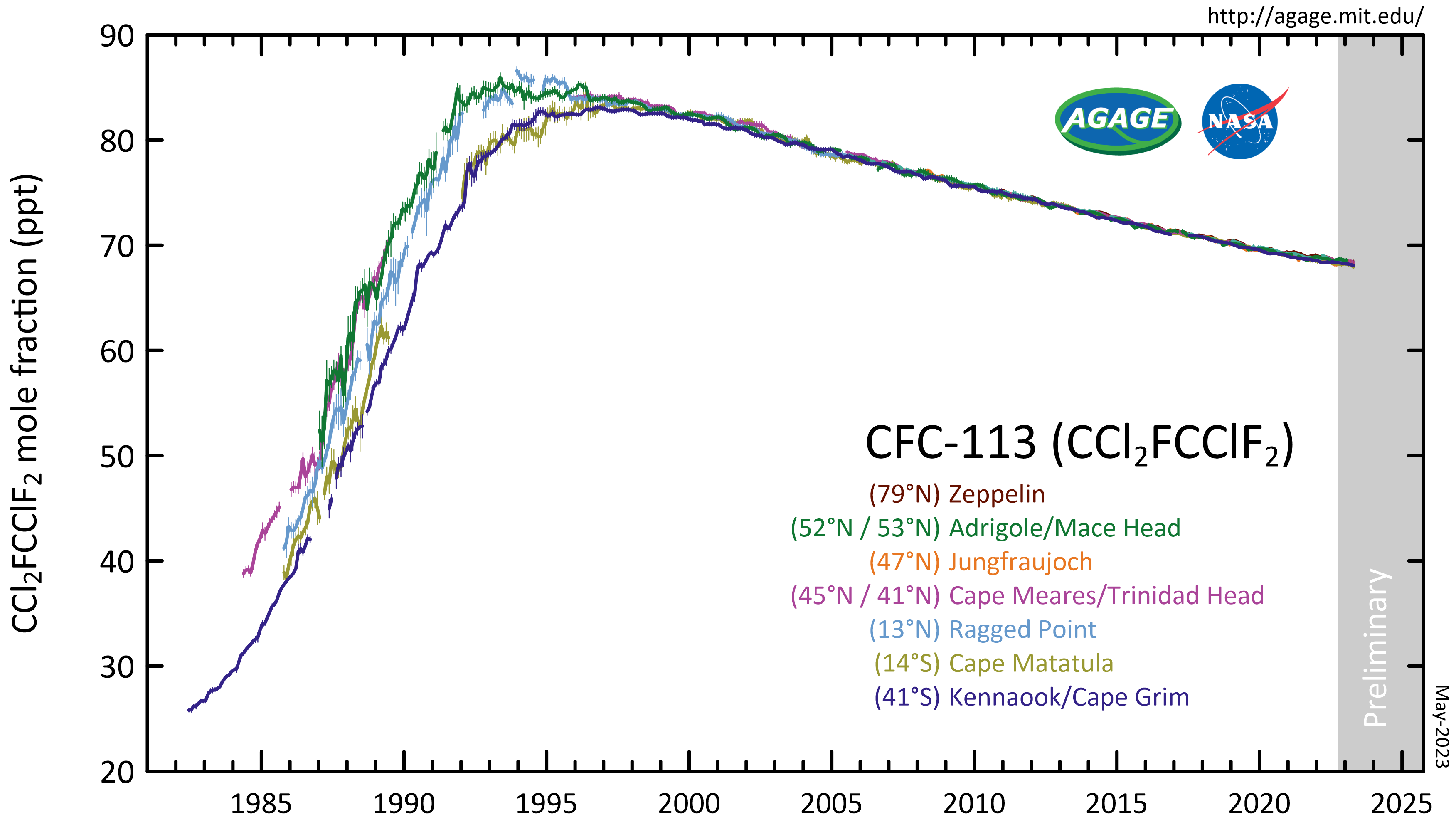
CFC-113
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane (often abbreviated as TCTFE) or CFC-113, is a chlorofluorocarbon. It has the formula . This colorless, volatile liquid is a versatile solvent. Production CFC-113 can be prepared from hexachloroethane and hydrofluoric acid: : This reaction may require catalysts such as antimony, chromium, iron and alumina at high temperatures. Another synthesis method uses HF on tetrachloroethylene instead. Atmospheric reactions CFC-113 is a very unreactive chlorofluorocarbon. It may remain in the atmosphere up to 90 years, sufficiently long that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation (UV, sunlight in the 190-225 nm range), generating chlorine radicals (Cl•), which initiate degradation of ozone requiring only a few minutes: : : This reaction is followed by: : The process regenerates Cl• to destroy more . The Cl• wil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kauri-butanol Value
The kauri-butanol value ("Kb value") is an international, standardized measure of solvent power for a hydrocarbon solvent, and is governed by an ASTM standardized test, ASTM D1133.ASTM D1133 - 10 Standard Test Method for Kauri-Butanol Value of Hydrocarbon Solvents The result of this test is a scaleless index, usually referred to as the "Kb value". A higher Kb value means the solvent is more aggressive or active in the ability to dissolve certain materials. Mild solvents have low scores in the tens and twenties; powerful solvents like chlorinated solvents and naphthenic aromatic solvents (i.e. "High Sol 10", "High Sol 15") have ratings that are in the low hundreds. In terms of the test itself, the kauri-butanol value (Kb) of a chemical shows the maximum amount of the hydrocarbon that can be added to a solution of kauri resin (a thick, gum-like material) in butanol (butyl alcohol) without causing cloudiness. Since kauri resin is readily soluble in butyl alcohol but not in most hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degreasing
Degreasing, often called defatting or fat trimming, is the removal of fatty acids from an object. In culinary science, degreasing is done with the intention of reducing the fat content of a meal. Degreasing food Degreasing is often used by dieters, particularly those following low-fat diets to reduce their fat consumption to induce weight loss. The energy content of 1 g of fat is 9 calories, while that of carbohydrates and proteins are 4 calories. Hence, dieters often view decreasing fat consumption as an efficient way of losing weight without greatly sacrificing total volume of food. Degreasing during meal preparation is used to reduce the energy content of the food being prepared. Those people who wish to reduce their cholesterol level or fat intake, in particular people with hypercholesterolemia often use degreasing to reduce their fat consumption. Degreasing of a meal during preparation Fat trimming of a meal can be done during preparation by a variety of methods. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bitumen
Bitumen ( , ) is an immensely viscosity, viscous constituent of petroleum. Depending on its exact composition, it can be a sticky, black liquid or an apparently solid mass that behaves as a liquid over very large time scales. In American English, the material is commonly referred to as asphalt or tar. Whether found in natural deposits or refined from petroleum, the substance is classed as a pitch (resin), pitch. Prior to the 20th century, the term asphaltum was in general use. The word derives from the Ancient Greek word (), which referred to natural bitumen or pitch. The largest natural deposit of bitumen in the world is the Pitch Lake of southwest Trinidad, which is estimated to contain 10 million tons. About 70% of annual bitumen production is destined for road surface, road construction, its primary use. In this application, bitumen is used to bind construction aggregate, aggregate particles like gravel and forms a substance referred to as asphalt concrete, which is collo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adhesive
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation. The use of adhesives offers certain advantages over other binding techniques such as sewing, mechanical fastenings, and welding. These include the ability to bind different materials together, the more efficient distribution of stress across a joint, the cost-effectiveness of an easily mechanized process, and greater flexibility in design. Disadvantages of adhesive use include decreased stability at high temperatures, relative weakness in bonding large objects with a small bonding surface area, and greater difficulty in separating objects during testing. Adhesives are typically organized by the method of adhesion followed by ''reactive'' or ''non-reactive'', a term which refers to whether the adhesive chemically reacts in order to harden. Alternatively, they can be organized either ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |