|
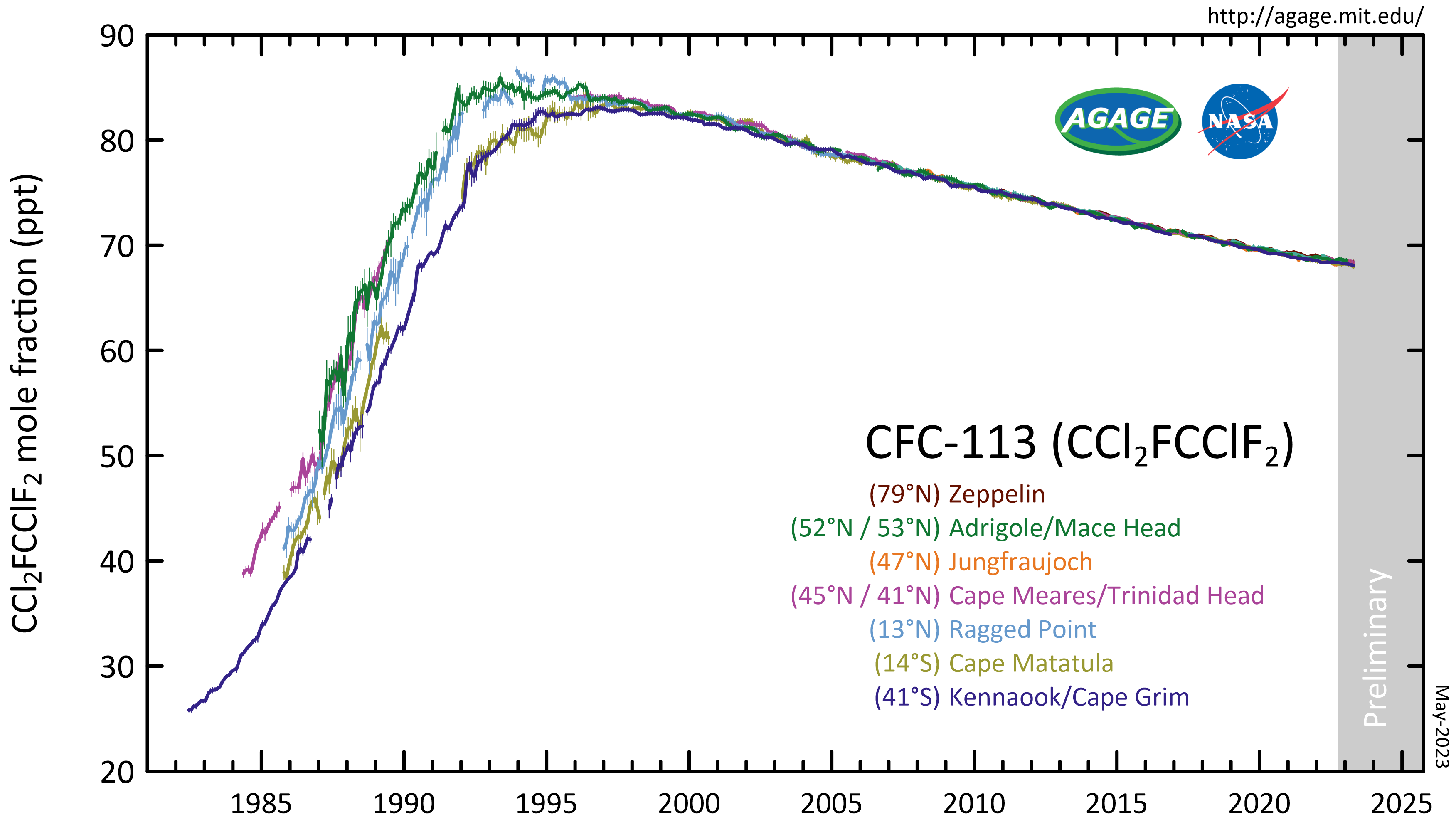
CFC-113
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane (often abbreviated as TCTFE) or CFC-113, is a chlorofluorocarbon. It has the formula . This colorless, volatile liquid is a versatile solvent. Production CFC-113 can be prepared from hexachloroethane and hydrofluoric acid: : This reaction may require catalysts such as antimony, chromium, iron and alumina at high temperatures. Another synthesis method uses HF on tetrachloroethylene instead. Atmospheric reactions CFC-113 is a very unreactive chlorofluorocarbon. It may remain in the atmosphere up to 90 years, sufficiently long that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation (UV, sunlight in the 190-225 nm range), generating chlorine radicals (Cl•), which initiate degradation of ozone requiring only a few minutes: : : This reaction is followed by: : The process regenerates Cl• to destroy more . The Cl• wil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly Halogenation, halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatility (chemistry), volatile derivatives of methane, ethane, and propane. The most common example of a CFC is dichlorodifluoromethane (R-12). R-12, also commonly called Freon, is used as a refrigerant. Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), gaseous fire suppression systems, and solvents. As a result of CFCs contributing to ozone depletion in the upper atmosphere, the manufacture of such compounds has been phased out under the Montreal Protocol, and they are being replaced with other products such as hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs) including R-410A, R-134a and 2,3,3,3-Tetrafluoropropene, R-1234yf. Structure, properties and production As in simpler alkanes, carbons in CFCs bond with tetrahe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,1-Trichloro-2,2,2-trifluoroethane
1,1,1-Trichloro-2,2,2-trifluoroethane, also called Asymmetrical trichlorotrifluoroethane or CFC-113a, is a chlorofluorocarbon (CFC) with the formula . Ozone depletion A team of researchers at the University of East Anglia analysed unpolluted air samples from Tasmania dating from the period 1978 to 2012. They concluded that the CFCs they studied had started entering the atmosphere from anthropogenic sources in the 1960s and that while the abundance of certain CFCs had decreased, owing to the Montreal Protocol, the abundance of CFC-113a in the atmosphere was still growing. Its main source remained uncertain, but production of hydrofluorocarbons Hydrofluorocarbons (HFCs) are synthetic organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air condit ... in East Asia was suspected. Between 2012 and 2017, concentrations of the gas jumped by 40 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry-cleaning
Dry cleaning is any cleaning process for clothing and textiles using a solvent other than water. Clothes are instead soaked in a water-free liquid solvent (usually non-polar, as opposed to water which is a polar solvent). Perchloroethylene (known in the industry as "perc") is the most commonly used solvent, although alternative solvents such as hydrocarbons, and decamethylcyclopentasiloxane are also used. Most natural fibers can be washed in water but some synthetics (e.g., viscose) react poorly with water and should be dry cleaned if possible. If not, this could result in changes in texture, strength, and shape. Additionally, certain specialty fabrics, including silk and rayon, may also benefit from dry cleaning to prevent damage. History The ancient Greeks and Romans had some waterless methods to clean textiles, involving the use of powdered chemicals and absorbent clay ( fuller's earth). By the 1700s, the French were using turpentine-based solvents for specialized clea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachloroethylene
Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc (or PERC), and PCE, is a chlorocarbon with the formula . It is a non-flammable, stable, colorless and heavy liquid widely used for dry cleaning of fabrics and occasionally as a highly effective automotive brake cleaner. It has a mildly sweet, sharp odor, detectable by most people at a concentration of 50 ppm. Tetrachloroethylene is regarded as a toxic substance, a human health hazard, and an environmental hazard. In 2020, the United States Environmental Protection Agency stated that "tetrachloroethylene exposure may harm the nervous system, liver, kidneys, and reproductive system, and may be harmful to unborn children", and reported that numerous toxicology agencies regard it as a carcinogen. History and production French chemist Henri Victor Regnault first synthesized tetrachloroethylene in 1839 by thermal decomposition of hexachloroethane f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a non-flammable, dense, colourless liquid with a "sweet" chloroform-like odour that can be detected at low levels. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, an anthelmintic and a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal. Properties In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedron, tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetric geometry, CCl4 is non-polar. methane, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachloroethylene
Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc (or PERC), and PCE, is a chlorocarbon with the formula . It is a non-flammable, stable, colorless and heavy liquid widely used for dry cleaning of fabrics and occasionally as a highly effective automotive brake cleaner. It has a mildly sweet, sharp odor, detectable by most people at a concentration of 50 ppm. Tetrachloroethylene is regarded as a toxic substance, a human health hazard, and an environmental hazard. In 2020, the United States Environmental Protection Agency stated that "tetrachloroethylene exposure may harm the nervous system, liver, kidneys, and reproductive system, and may be harmful to unborn children", and reported that numerous toxicology agencies regard it as a carcinogen. History and production French chemist Henri Victor Regnault first synthesized tetrachloroethylene in 1839 by thermal decomposition of hexachloroethane f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Cleaning
Dry cleaning is any cleaning process for clothing and textiles using a solvent other than water. Clothes are instead soaked in a water-free liquid solvent (usually non-polar, as opposed to water which is a Solvent#Solvent classifications, polar solvent). Perchloroethylene (known in the industry as "perc") is the most commonly used solvent, although alternative solvents such as hydrocarbons, and decamethylcyclopentasiloxane are also used. Most natural fibers can be washed in water but some synthetics (e.g., viscose) react poorly with water and should be dry cleaned if possible. If not, this could result in changes in texture, strength, and shape. Additionally, certain specialty fabrics, including silk and rayon, may also benefit from dry cleaning to prevent damage. History The ancient Greeks and Romans had some waterless methods to clean textiles, involving the use of powdered chemicals and absorbent clay (fuller's earth). By the 1700s, the French were using turpentine-based s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lexington, Massachusetts
Lexington is a suburban town in Middlesex County, Massachusetts, United States, located 10 miles (16 km) from Downtown Boston. The population was 34,454 as of the 2020 United States census, 2020 census. The area was originally inhabited by Native Americans, and was first settled by Europeans as a farming community. Lexington is well known as the site of the first shots of the American Revolutionary War, in the Battles of Lexington and Concord, Battle of Lexington on April 19, 1775, where the "Shot heard round the world, Shot heard 'round the world" took place. It is home to Minute Man National Historical Park. History Indigenous history Native Americans in the United States, Native Americans inhabited the area that would become Lexington for thousands of years prior to European colonization of the Americas, as attested by a woodland era archaeological site near Loring Hill south of the town center. At the time of European contact, the area may have been a border region ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CFC-12
Dichlorodifluoromethane (R-12) is a colorless gas popularly known by the genericized brand name Freon (as Freon-12). It is a chlorofluorocarbon halomethane (CFC) used as a refrigerant and aerosol spray propellant. In compliance with the Montreal Protocol, its manufacture was banned in developed countries (non-article 5 countries) in 1996, and in developing countries (Article 5 countries) in 2010 out of concerns about its damaging effect on the ozone layer. Its only allowed usage is as a fire retardant in submarines and aircraft. It is soluble in many organic solvents. R-12 cylinders are colored white. Preparation It can be prepared by reacting carbon tetrachloride with hydrogen fluoride in the presence of a catalytic amount of antimony pentachloride: :CCl4 + 2HF → CCl2F2 + 2HCl This reaction can also produce trichlorofluoromethane (CCl3F), chlorotrifluoromethane (CClF3) and tetrafluoromethane (CF4). History Charles F. Kettering, vice president of General Motors Research C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorotrifluoroethylene
Chlorotrifluoroethylene (CTFE) is a chlorofluorocarbon with chemical formula CFCl=CF2. It is commonly used as a refrigerant in cryogenic applications. CTFE has a carbon-carbon double bond and so can be polymerized to form polychlorotrifluoroethylene or copolymerized to produce the plastic ECTFE. PCTFE has the trade name Neoflon PCTFE from Daikin Industries in Japan, and it used to be produced under the trade name Kel-F from 3M Corporation in Minnesota. Production and reactions Chlorotrifluoroethylene is produced commercially by the dechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane with zinc: :CFCl2-CF2Cl + Zn → CClF=CF2 + ZnCl2 In 2012, an estimated 1–10 million pounds were produced commercially in the United States. Addition of iodine monochloride to chlorotrifluoroethylene gives iododichlorotrifluoroethane: : The latter is a precursor to hexafluorobutadiene. Thermal dimerization of chlorotrifluoroethylene gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosgene
Phosgene is an organic chemical compound with the formula . It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. It can be thought of chemically as the double acyl chloride analog of carbonic acid, or structurally as formaldehyde with the hydrogen atoms replaced by chlorine atoms. In 2013, about 75–80 % of global phosgene was consumed for isocyanates, 18% for polycarbonates and about 5% for other fine chemicals. Phosgene is extremely poisonous and was used as a chemical weapon during World War I, where it was responsible for 85,000 deaths. It is a highly potent pulmonary irritant and quickly filled enemy trenches due to it being a heavy gas. It is classified as a Schedule 3 substance under the Chemical Weapons Convention. In addition to its industrial production, small amounts occur from the breakdown and the combustion of organochlorine compounds, such as chloroform. Structure and basic properties Phosgene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |