|
Kasha–Vavilov Rule
Kasha's rule is a principle in the photochemistry of electronically excited molecules. The rule states that photon emission (fluorescence or phosphorescence) occurs in appreciable yield only from the lowest excited state of a given multiplicity. It is named after American spectroscopist Michael Kasha, who proposed it in 1950. Description and explanation The rule is relevant in understanding the emission spectrum of an excited molecule. Upon absorbing a photon, a molecule in its electronic ground state (denoted ''S''0, assuming a singlet state) may – depending on the photon wavelength – be excited to any of a set of higher electronic states (denoted ''S''n where ''n''>0). However, according to Kasha's rule, photon emission (termed fluorescence in the case of an ''S'' state) is expected in appreciable yield only from the lowest excited state, ''S''1. Since only one state is expected to yield emission, an equivalent statement of the rule is that the emission wavelength is in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Franck–Condon Principle
The Franck–Condon principle describes the intensities of vibronic transitions, or the absorption or emission of a photon. It states that when a molecule is undergoing an electronic transition, such as ionization, the nuclear configuration of the molecule experiences no significant change. Overview The Franck–Condon principle has a well-established semiclassical interpretation based on the original contributions of James Franck. Electronic transitions are relatively instantaneous compared with the time scale of nuclear motions, therefore if the molecule is to move to a new vibrational level during the electronic transition, this new vibrational level must be instantaneously compatible with the nuclear positions and momenta of the vibrational level of the molecule in the originating electronic state. In the semiclassical picture of vibrations (oscillations) of a simple harmonic oscillator, the necessary conditions can occur at the turning points, where the momentum is zer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Yield
In particle physics, the quantum yield (denoted ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system. \Phi(\lambda)=\frac Applications Fluorescence spectroscopy The fluorescence quantum yield is defined as the ratio of the number of photons emitted to the number of photons absorbed.Lakowicz, Joseph R. ''Principles of Fluorescence Spectroscopy'' (Kluwer Academic / Plenum Publishers 1999) p.10. \Phi = \frac Fluorescence quantum yield is measured on a scale from 0 to 1.0, but is often represented as a percentage. A quantum yield of 1.0 (100%) describes a process where each photon absorbed results in a photon emitted. Substances with the largest quantum yields, such as rhodamines, display the brightest emissions; however, compounds with quantum yields of 0.10 are still considered quite fluorescent. Quantum yield is defined by the fraction of excited state fluorophores that decay through fluorescence: \Phi_f = \f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sergey Ivanovich Vavilov
Sergey Ivanovich Vavilov ( ; – January 25, 1951) was a Soviet Union, Soviet physicist, the President of the Academy of Sciences of the Soviet Union from July 1945 until his death. His elder brother Nikolai Vavilov was a famous Russian geneticist. Biography Vavilov founded the Soviet school of physical optics, known by his works in luminescence. In 1934 he co-discovered the Cherenkov radiation, Vavilov-Cherenkov effect, a discovery for which Pavel Cherenkov was awarded a Nobel Prize in Physics in 1958. The Kasha's rule#Kasha–Vavilov rule, Kasha–Vavilov rule of luminescence quantum yields is also named for him. He was a member of the USSR Academy of Sciences from 1932, Head of the Lebedev Physical Institute, Lebedev Institute of Physics (since 1934), a chief editor of the ''Great Soviet Encyclopedia'', a member of the Supreme Soviet from 1946 and a recipient of four USSR State Prize, Stalin Prizes (1943, 1946, 1951, 1952). He wrote on the lives and works of great thinker ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfaction, olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of Resonance (chemistry), resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-covalent bond, bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Friedrich August Kekulé ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiaromatic
Antiaromaticity is a chemical property of a cyclic molecule with a π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised (π or lone pair) electrons in it, as opposed to aromaticity. Unlike aromatic compounds, which follow Hückel's rule ( ''n''+2π electrons) and are highly stable, antiaromatic compounds are highly unstable and highly reactive. To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy. Examples of antiaromatic compounds are pentalene (A), biphenylene (B), cyclopentadienyl cation (C). The prototypical example of antiaromaticity, cyclobutadiene, is the subject of debate, with some scientists arguing that antiaromaticity is not a major factor contributing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azulene
Azulene is an aromatic organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. The compound is named after its colour, as "azul" is Spanish for blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates. Azulene has a long history, dating back to the 15th century as the azure-blue chromophore obtained by steam distillation of German chamomile. The chromophore was discovered in yarrow and wormwood and named in 1863 by Septimus Piesse. Its structure was first reported by Lavoslav Ružička, followed by its organic synthesis in 1937 by Placidus Plattner. Structure and bonding left, The blue color of the mushroom '' Lactarius indigo'' is due to the azulene derivative (7-isopropenyl-4-methylazulen-1-yl)methyl stearate. Azulene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
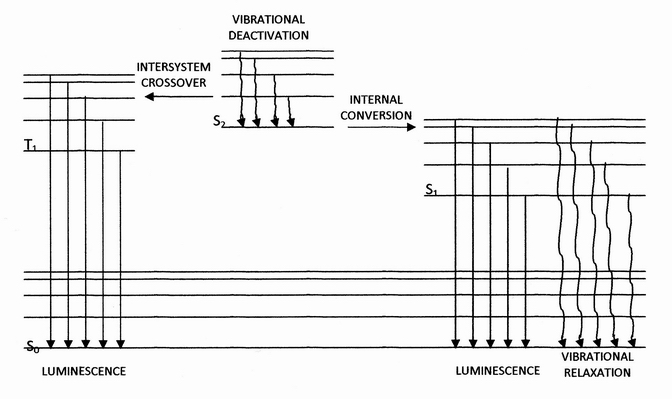
Internal Conversion (chemistry)
Internal conversion is a transition from a higher to a lower electronic state in a molecule or atom.A general and quantitative discussion of intramolecular radiationless transitions is the subject of an article by M. Bixon and J. Jortner (''J. Chem. Phys.'', 48 (2) 715-726 (1968)). It is sometimes called "radiationless de-excitation", because no photons are emitted. It differs from intersystem crossing in that, while both are radiationless methods of de-excitation, the molecular spin state for internal conversion remains the same, whereas it changes for intersystem crossing. The energy of the electronically excited state is given off to vibrational modes of the molecule. The excitation energy is transformed into heat. Examples A classic example of this process is the quinine sulfate fluorescence, which can be quenched by the use of various halide salts. The excited molecule can de-excite by increasing the thermal energy of the surrounding solvated ions. Several natural molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wavefunction
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter), psi, respectively). Wave functions are complex number, complex-valued. For example, a wave function might assign a complex number to each point in a region of space. The Born rule provides the means to turn these complex probability amplitudes into actual probabilities. In one common form, it says that the squared modulus of a wave function that depends upon position is the probability density function, probability density of measurement in quantum mechanics, measuring a particle as being at a given place. The integral of a wavefunction's squared modulus over all the system's degrees of freedom must be equal to 1, a condition called ''normalization''. Since the wave function is complex-valued, only its relative phase and relative magnitud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Numbers
In Quantum mechanics, quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. To fully specify the state of the electron in a hydrogen atom, four quantum numbers are needed. The traditional set of quantum numbers includes the Principal quantum number, principal, Azimuthal quantum number, azimuthal, Magnetic quantum number, magnetic, and Spin quantum number, spin quantum numbers. To describe other systems, different quantum numbers are required. For subatomic particles, one needs to introduce new quantum numbers, such as the flavour (particle physics), flavour of quarks, which have no classical correspondence. Quantum numbers are closely related to eigenvalues of observables. When the corresponding observable commutes with the Hamiltonian (quantum mechanics), Hamiltonian of the system, the quantum number is said to be "Good quantum number, good", and acts as a constant of motion in the quantum dynamics. History Elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |