Internal conversion (chemistry) on:
[Wikipedia]
[Google]
[Amazon]
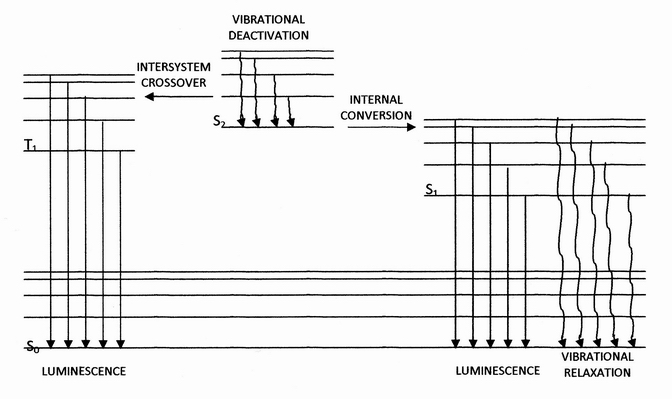 Internal conversion is a transition from a higher to a lower electronic state in a molecule or atom.A general and quantitative discussion of intramolecular radiationless transitions is the subject of an article by M. Bixon and J. Jortner (''J. Chem. Phys.'', 48 (2) 715-726 (1968)). It is sometimes called "radiationless de-excitation", because no
Internal conversion is a transition from a higher to a lower electronic state in a molecule or atom.A general and quantitative discussion of intramolecular radiationless transitions is the subject of an article by M. Bixon and J. Jortner (''J. Chem. Phys.'', 48 (2) 715-726 (1968)). It is sometimes called "radiationless de-excitation", because no
 Internal conversion is a transition from a higher to a lower electronic state in a molecule or atom.A general and quantitative discussion of intramolecular radiationless transitions is the subject of an article by M. Bixon and J. Jortner (''J. Chem. Phys.'', 48 (2) 715-726 (1968)). It is sometimes called "radiationless de-excitation", because no
Internal conversion is a transition from a higher to a lower electronic state in a molecule or atom.A general and quantitative discussion of intramolecular radiationless transitions is the subject of an article by M. Bixon and J. Jortner (''J. Chem. Phys.'', 48 (2) 715-726 (1968)). It is sometimes called "radiationless de-excitation", because no photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s are emitted. It differs from intersystem crossing
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.
Excited singlet and triplet states
When an electron in a molecule with a singlet grou ...
in that, while both are radiationless methods of de-excitation, the molecular spin state for internal conversion remains the same, whereas it changes for intersystem crossing.
The energy of the electronically excited state is given off to vibrational modes of the molecule. The excitation energy is transformed into heat.
Examples
A classic example of this process is thequinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg ...
sulfate fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
, which can be quenched by the use of various halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
s. The excited molecule can de-excite by increasing the thermal energy of the surrounding solvated ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s.
Several natural molecules perform a fast internal conversion. This ability to transform the excitation energy of photon into heat can be a crucial property for photoprotection
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative str ...
by molecules such as melanin
Melanin (; ) is a family of biomolecules organized as oligomers or polymers, which among other functions provide the pigments of many organisms. Melanin pigments are produced in a specialized group of cells known as melanocytes.
There are ...
. Fast internal conversion reduces the excited state lifetime, and thereby prevents bimolecular
In chemistry, molecularity is the number of molecules that come together to react in an elementary reaction, elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of Sto ...
reactions. Bimolecular electron transfer always produces a reactive chemical species, free radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired electron, unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemical reaction, chemi ...
. Nucleic acids (precisely the single, free nucleotides, not those bound in a DNA/RNA strand) have an extremely short lifetime due to a fast internal conversion.
Both melanin and DNA have some of the fastest internal conversion rates.
In applications that make use of bimolecular electron transfer the internal conversion is undesirable. For example, it is advantageous to have a long-lived excited state in Grätzel cells (Dye-sensitized solar cells).
See also
*Fluorescence spectroscopy
Fluorescence spectroscopy (also known as fluorimetry or spectrofluorometry) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electro ...
*Förster resonance energy transfer
Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores). ...
References
{{reflist Photochemistry Spectroscopy Fluorescence