 |
Wavefunction
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter), psi, respectively). Wave functions are complex number, complex-valued. For example, a wave function might assign a complex number to each point in a region of space. The Born rule provides the means to turn these complex probability amplitudes into actual probabilities. In one common form, it says that the squared modulus of a wave function that depends upon position is the probability density function, probability density of measurement in quantum mechanics, measuring a particle as being at a given place. The integral of a wavefunction's squared modulus over all the system's degrees of freedom must be equal to 1, a condition called ''normalization''. Since the wave function is complex-valued, only its relative phase and relative magnitud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Interpretations Of Quantum Mechanics
An interpretation of quantum mechanics is an attempt to explain how the mathematical theory of quantum mechanics might correspond to experienced reality. Quantum mechanics has held up to rigorous and extremely precise tests in an extraordinarily broad range of experiments. However, there exist a number of contending schools of thought over their interpretation. These views on interpretation differ on such fundamental questions as whether quantum mechanics is determinism, deterministic or stochastic, Principle_of_locality, local or Quantum_nonlocality, non-local, which elements of quantum mechanics can be considered real, and what the nature of measurement in quantum mechanics, measurement is, among other matters. While some variation of the Copenhagen interpretation is commonly presented in textbooks, many other interpretations have been developed. Despite nearly a century of debate and experiment, no consensus has been reached among physicists and Philosophy of physics, philosophe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Indeterminacy Principle
The uncertainty principle, also known as Heisenberg's indeterminacy principle, is a fundamental concept in quantum mechanics. It states that there is a limit to the precision with which certain pairs of physical properties, such as position and momentum, can be simultaneously known. In other words, the more accurately one property is measured, the less accurately the other property can be known. More formally, the uncertainty principle is any of a variety of mathematical inequalities asserting a fundamental limit to the product of the accuracy of certain related pairs of measurements on a quantum system, such as position, ''x'', and momentum, ''p''. Such paired-variables are known as complementary variables or canonically conjugate variables. First introduced in 1927 by German physicist Werner Heisenberg, the formal inequality relating the standard deviation of position ''σx'' and the standard deviation of momentum ''σp'' was derived by Earle Hesse Kennard later that ye ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Schrödinger Equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after Erwin Schrödinger, an Austrian physicist, who postulated the equation in 1925 and published it in 1926, forming the basis for the work that resulted in his Nobel Prize in Physics in 1933. Conceptually, the Schrödinger equation is the quantum counterpart of Newton's second law in classical mechanics. Given a set of known initial conditions, Newton's second law makes a mathematical prediction as to what path a given physical system will take over time. The Schrödinger equation gives the evolution over time of the wave function, the quantum-mechanical characterization of an isolated physical system. The equation was postulated by Schrödinger based on a postulate of Louis de Broglie that all matter has an associated matter wave. The equati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Spin (physics)
Spin is an Intrinsic and extrinsic properties, intrinsic form of angular momentum carried by elementary particles, and thus by List of particles#Composite particles, composite particles such as hadrons, atomic nucleus, atomic nuclei, and atoms. Spin is quantized, and accurate models for the interaction with spin require relativistic quantum mechanics or quantum field theory. The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum. The relativistic spin–statistics theorem connects electron spin quantization to the Pauli exclusion principle: observations of exclusion imply half-integer spin, and observations of half-integer spin imply exclusion. Spin is described mathematically as a vector for some particles such as photons, and as a spinor or bispinor for other particles such as electrons. Sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Probability Amplitude
In quantum mechanics, a probability amplitude is a complex number used for describing the behaviour of systems. The square of the modulus of this quantity at a point in space represents a probability density at that point. Probability amplitudes provide a relationship between the quantum state vector of a system and the results of observations of that system, a link that was first proposed by Max Born, in 1926. Interpretation of values of a wave function as the probability amplitude is a pillar of the Copenhagen interpretation of quantum mechanics. In fact, the properties of the space of wave functions were being used to make physical predictions (such as emissions from atoms being at certain discrete energies) before any physical interpretation of a particular function was offered. Born was awarded half of the 1954 Nobel Prize in Physics for this understanding, and the probability thus calculated is sometimes called the "Born probability". These probabilistic concepts, namel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Operator (quantum Mechanics)
An operator is a function over a space of physical states onto another space of states. The simplest example of the utility of operators is the study of symmetry (which makes the concept of a group useful in this context). Because of this, they are useful tools in classical mechanics. Operators are even more important in quantum mechanics, where they form an intrinsic part of the formulation of the theory. They play a central role in describing observables (measurable quantities like energy, momentum, etc.). Operators in classical mechanics In classical mechanics, the movement of a particle (or system of particles) is completely determined by the Lagrangian L(q, \dot, t) or equivalently the Hamiltonian H(q, p, t), a function of the generalized coordinates ''q'', generalized velocities \dot = \mathrm q / \mathrm t and its conjugate momenta: :p = \frac If either ''L'' or ''H'' is independent of a generalized coordinate ''q'', meaning the ''L'' and ''H'' do not change when ''q' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Fourier Transform
In mathematics, the Fourier transform (FT) is an integral transform that takes a function as input then outputs another function that describes the extent to which various frequencies are present in the original function. The output of the transform is a complex-valued function of frequency. The term ''Fourier transform'' refers to both this complex-valued function and the mathematical operation. When a distinction needs to be made, the output of the operation is sometimes called the frequency domain representation of the original function. The Fourier transform is analogous to decomposing the sound of a musical chord into the intensities of its constituent pitches. Functions that are localized in the time domain have Fourier transforms that are spread out across the frequency domain and vice versa, a phenomenon known as the uncertainty principle. The critical case for this principle is the Gaussian function, of substantial importance in probability theory and statist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Quantum Physics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is the foundation of all quantum physics, which includes quantum chemistry, quantum field theory, quantum technology, and quantum information science. Quantum mechanics can describe many systems that classical physics cannot. Classical physics can describe many aspects of nature at an ordinary (macroscopic and Microscopic scale, (optical) microscopic) scale, but is not sufficient for describing them at very small submicroscopic (atomic and subatomic) scales. Classical mechanics can be derived from quantum mechanics as an approximation that is valid at ordinary scales. Quantum systems have Bound state, bound states that are Quantization (physics), quantized to Discrete mathematics, discrete values of energy, momentum, angular momentum, and ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up quark, up and down quark, down quarks. Electrons are extremely lightweight particles that orbit the positively charged atomic nucleus, nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall electric charge#Charge neutrality, neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electron, valence electrons are the least tightly bound and are responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Measurement In Quantum Mechanics
In quantum physics, a measurement is the testing or manipulation of a physical system to yield a numerical result. A fundamental feature of quantum theory is that the predictions it makes are probabilistic. The procedure for finding a probability involves combining a quantum state, which mathematically describes a quantum system, with a mathematical representation of the measurement to be performed on that system. The formula for this calculation is known as the Born rule. For example, a quantum particle like an electron can be described by a quantum state that associates to each point in space a complex number called a probability amplitude. Applying the Born rule to these amplitudes gives the probabilities that the electron will be found in one region or another when an experiment is performed to locate it. This is the best the theory can do; it cannot say for certain where the electron will be found. The same quantum state can also be used to make a prediction of how the electro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
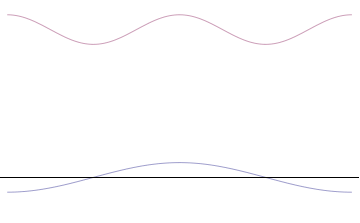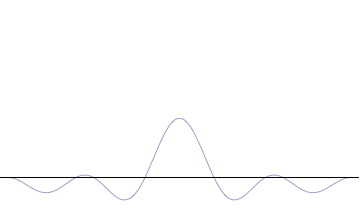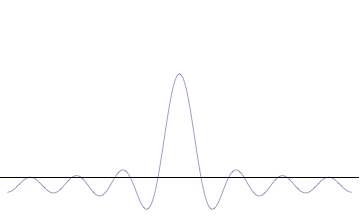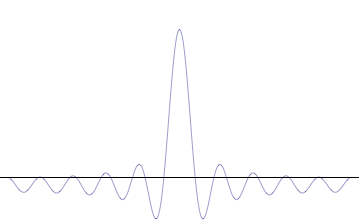
Wave
In physics, mathematics, engineering, and related fields, a wave is a propagating dynamic disturbance (change from List of types of equilibrium, equilibrium) of one or more quantities. ''Periodic waves'' oscillate repeatedly about an equilibrium (resting) value at some frequency. When the entire waveform moves in one direction, it is said to be a travelling wave; by contrast, a pair of superposition principle, superimposed periodic waves traveling in opposite directions makes a ''standing wave''. In a standing wave, the amplitude of vibration has nulls at some positions where the wave amplitude appears smaller or even zero. There are two types of waves that are most commonly studied in classical physics: mechanical waves and electromagnetic waves. In a mechanical wave, Stress (mechanics), stress and Strain (mechanics), strain fields oscillate about a mechanical equilibrium. A mechanical wave is a local deformation (physics), deformation (strain) in some physical medium that propa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |