|
Isotopes Of Phosphorus
Although phosphorus (15P) has 22 isotopes from 26P to 47P, only 31P is stable, thus phosphorus is considered a monoisotopic element. The longest-lived radioactive isotopes are 33P with a half-life of 25.34 days and 32P with a half-life of 14.268 days. All others have half-lives of under 2.5 minutes, most under a second. The least stable known isotope is 47P, with a half-life of 2 milliseconds. List of isotopes , -id=Phosphorus-26 , rowspan=3, 26P , rowspan=3 style="text-align:right" , 15 , rowspan=3 style="text-align:right" , 11 , rowspan=3, 26.01178(21)# , rowspan=3, 43.6(3) ms , β+ (62.9%) , 26Si , rowspan=3, (3)+ , rowspan=3, , - , β+, p (35.1%) , 25Al , - , β+, 2p (1.99%) , 24Mg , -id=Phosphorus-26m , style="text-indent:1em", 26mP , colspan=3 style="text-indent:2em", 164.4(1) keV , 115(8) ns , IT , 26P , (1+) , , -id=Phosphorus-27 , rowspan=2, 27P , rowspan=2 style="text-align:right" , 15 , rowspan=2 style="text-align:right" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared artificially, the two most common allotropes being white phosphorus and red phosphorus. With as its only stable isotope, phosphorus has an occurrence in Earth's crust of about 0.1%, generally as phosphate rock. A member of the pnictogen family, phosphorus readily forms a wide variety of organic compound, organic and inorganic compound, inorganic compounds, with as its main oxidation states +5, +3 and −3. The isolation of white phosphorus in 1669 by Hennig Brand marked the scientific community's first discovery since Antiquity of an element. The name phosphorus is a reference to the Phosphorus (morning star), god of the Morning star in Greek mythology, inspired by the faint glow of white phosphorus when exposed to oxygen. This property is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Error
In statistics and optimization, errors and residuals are two closely related and easily confused measures of the deviation of an observed value of an element of a statistical sample from its "true value" (not necessarily observable). The error of an observation is the deviation of the observed value from the true value of a quantity of interest (for example, a population mean). The residual is the difference between the observed value and the '' estimated'' value of the quantity of interest (for example, a sample mean). The distinction is most important in regression analysis, where the concepts are sometimes called the regression errors and regression residuals and where they lead to the concept of studentized residuals. In econometrics, "errors" are also called disturbances. Introduction Suppose there is a series of observations from a univariate distribution and we want to estimate the mean of that distribution (the so-called location model (statistics), location model). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Phosphorus
Although phosphorus (15P) has 22 isotopes from 26P to 47P, only 31P is stable, thus phosphorus is considered a monoisotopic element. The longest-lived radioactive isotopes are 33P with a half-life of 25.34 days and 32P with a half-life of 14.268 days. All others have half-lives of under 2.5 minutes, most under a second. The least stable known isotope is 47P, with a half-life of 2 milliseconds. List of isotopes , -id=Phosphorus-26 , rowspan=3, 26P , rowspan=3 style="text-align:right" , 15 , rowspan=3 style="text-align:right" , 11 , rowspan=3, 26.01178(21)# , rowspan=3, 43.6(3) ms , β+ (62.9%) , 26Si , rowspan=3, (3)+ , rowspan=3, , - , β+, p (35.1%) , 25Al , - , β+, 2p (1.99%) , 24Mg , -id=Phosphorus-26m , style="text-indent:1em", 26mP , colspan=3 style="text-indent:2em", 164.4(1) keV , 115(8) ns , IT , 26P , (1+) , , -id=Phosphorus-27 , rowspan=2, 27P , rowspan=2 style="text-align:right" , 15 , rowspan=2 style="text-align:right" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
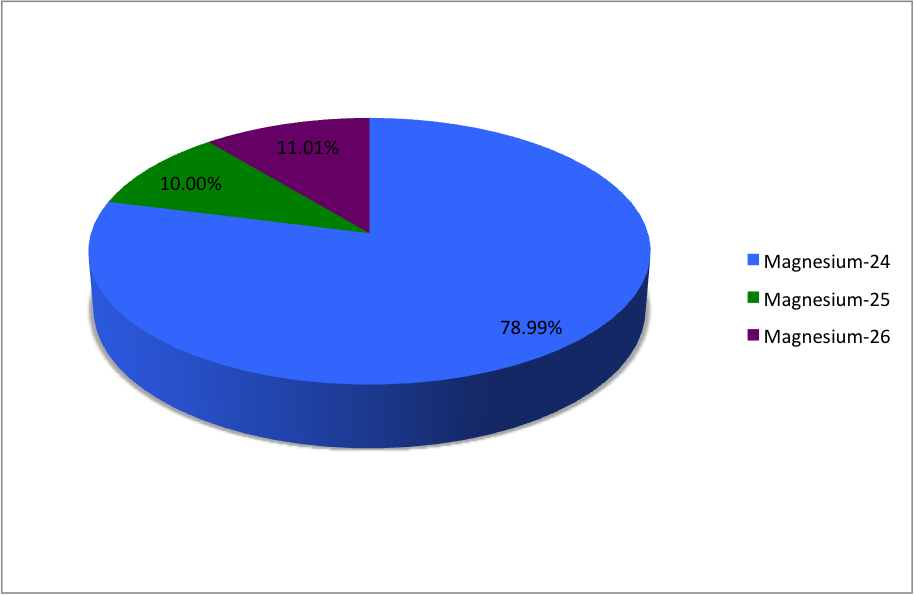
Isotopes Of Magnesium
Magnesium (12Mg) naturally occurs in three stable isotopes: , , and . There are 19 radioisotopes that have been discovered, ranging from to (with the exception of ). The longest-lived radioisotope is with a half-life of . The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. The shortest-lived is proton-unbound with a half-life of . A precise measurement of the neutron-rich 40Mg in 2019 showed the unexpected difference in its nuclear structure, compared to the lighter neighboring isotopes. List of isotopes , -id=Magnesium-18 , , style="text-align:right" , 12 , style="text-align:right" , 6 , , , 2p , , 0+ , , , -id=Magnesium-19 , , style="text-align:right" , 12 , style="text-align:right" , 7 , , , 2p , , 1/2−# , , , - , rowspan=2, , rowspan=2 style="text-align:right" , 12 , rowspan=2 style="text-align:right" , 8 , rowspan=2, , rowspan=2, , β+ () , , rowsp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Aluminum
Aluminium or ''aluminum'' (13Al) has 23 known isotopes from 21Al to 43Al and 4 known isomers. Only 27Al (stable isotope) and 26Al (radioactive isotope, t1/2 = ) occur naturally, however 27Al comprises nearly all natural aluminium. Other than 26Al, all radioisotopes have half-lives under 7 minutes, most under a second. The standard atomic weight is . 26Al is produced from argon in the atmosphere by spallation caused by cosmic-ray protons. Aluminium isotopes have found practical application in dating marine sediments, manganese nodules, glacial ice, quartz in rock exposures, and meteorites. The ratio of 26Al to 10Be has been used to study the role of sediment transport, deposition, and storage, as well as burial times, and erosion, on 105 to 106 year time scales. 26Al has also played a significant role in the study of meteorites. List of isotopes , -id=Aluminium-21 , 21Al , style="text-align:right" , 13 , style="text-align:right" , 8 , 21.0278(13) , >1.1 zs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Silicon
Silicon (14Si) has 25 known isotopes, with mass numbers ranging from 22 to 46. 28Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable. The longest-lived radioisotope is 32Si, which is produced by cosmic ray spallation of argon. Its half-life has been determined to be approximately 150 years (with decay energy 0.21 MeV), and it decays by beta emission to 32 P (which has a 14.27-day half-life) and then to 32 S. After 32Si, 31Si has the second longest half-life at 157.3 minutes. All others have half-lives under 7 seconds. List of isotopes , -id=Silicon-22 , rowspan=3, 22Si , rowspan=3 style="text-align:right" , 14 , rowspan=3 style="text-align:right" , 8 , rowspan=3, 22.03611(54)# , rowspan=3, 28.7(11) ms , β+, p (62%) , 21Mg , rowspan=3, 0+ , rowspan=3, , rowspan=3, , - , β+ (37%) , 22Al , - , β+, 2p (0.7%) , 20Na , -id=Silicon-23 , rowspan=3, 23Si , rowspan=3 style="text-align:right" , 14 , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Sulfur
Sulfur (16S) has 23 known isotopes with mass numbers ranging from 27 to 49, four of which are stable: 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%). The preponderance of sulfur-32 is explained by its production from carbon-12 plus successive fusion capture of five helium-4 nuclei, in the so-called alpha process of exploding type II supernovas (see silicon burning). Other than 35S, the radioactive isotopes of sulfur are all comparatively short-lived. 35S is formed from cosmic ray spallation of 40 Ar in the atmosphere. It has a half-life of 87 days. The next longest-lived radioisotope is sulfur-38, with a half-life of 170 minutes. Isotopes lighter than 32S mostly decay to isotopes of phosphorus or silicon, while 35S and heavier radioisotopes decay to isotopes of chlorine. The beams of several radioactive isotopes (such as those of 44S) have been studied theoretically within the framework of the synthesis of superheavy elements, especially those ones in the vicinity o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Isotope Biogeochemistry
Sulfur isotope biogeochemistry is the study of the distribution of isotopes of sulfur, sulfur isotopes in biological and geological materials. In addition to its common isotope, 32S, sulfur has three rare stable isotopes: 34S, 36S, and 33S. The distribution of these isotopes in the environment is controlled by many biochemical and physical processes, including biological metabolisms, mineral formation processes, and atmospheric chemistry. Measuring the abundance of sulfur stable isotopes in natural materials, like bacterial cultures, minerals, or seawater, can reveal information about these processes both in the modern environment and over Earth history. Background Natural abundance of sulfur isotopes Sulfur has 24 known isotopes, 4 of which are Stable nuclide, stable (meaning that they do not undergo radioactive decay). 32S, the common isotope of sulfur, makes up 95.0% of the natural sulfur on Earth. In the atomic symbol of 32S, the number 32 refers to the mass of each sulfur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Systematic Error
Observational error (or measurement error) is the difference between a measurement, measured value of a physical quantity, quantity and its unknown true value.Dodge, Y. (2003) ''The Oxford Dictionary of Statistical Terms'', OUP. Such errors are inherent in the measurement process; for example lengths measured with a ruler calibrated in whole centimeters will have a measurement error of several millimeters. The error or uncertainty of a measurement can be estimated, and is specified with the measurement as, for example, 32.3 ± 0.5 cm. Scientific observations are marred by two distinct types of errors, systematic errors on the one hand, and Statistical randomness, random, on the other hand. The effects of random errors can be mitigated by the repeated measurements. Constant or systematic errors on the contrary must be carefully avoided, because they arise from one or more causes which constantly act in the same way, and have the effect of always altering the result of the experi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a Atomic nucleus, nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photodisintegration, photoneutron emission and beta-delayed neutron emission. As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element. Neutrons are also produced in the spontaneous fission, spontaneous and nuclear fission, induced fission of certain heavy nuclides. Spontaneous neutron emission As a consequence of the Pauli exclusion principle, nuclei with an excess of protons or neutrons have a higher average energy per nucleon. Nuclei with a sufficient excess of neutrons have a greater energy than the combination of a free neutron and a nucleus with one less neutron, and therefore can decay by neutron emission. Nuclei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemical element), but different nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have similar chemical properties, they have different atomic masses and physical properties. The term isotope is derived from the Greek roots isos (wikt:ἴσος, ἴσος "equal") and topos (wikt:τόπος, τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd (doctor), Margaret Todd in a 1913 suggestion to the British chemist Frederick Soddy, who popularized the term. The number of protons within the atomic nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Radioisotope
A trace radioisotope is a radioisotope that occurs naturally in trace amounts (i.e. extremely small). Generally speaking, trace radioisotopes have half-lives that are short in comparison with the age of the Earth, since primordial nuclides tend to occur in larger than trace amounts. Trace radioisotopes are therefore present only because they are continually produced on Earth by natural processes. Natural processes which produce trace radioisotopes include cosmic ray bombardment of stable nuclides, ordinary alpha and beta decay of the long-lived heavy nuclides, thorium-232, uranium-238, and uranium-235, spontaneous fission of uranium-238, and nuclear transmutation Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed. A transmutat ... reactions induced by natural radioactivity, such as the production of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |