|
Hydroxyquinone
Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone or ''para''-benzoquinone (which often called just "quinone"). More generally, the term may refer to any derivative of any quinone (such as 1,2-benzoquinone, 1,4-naphthoquinone or 9,10-anthraquinone), where any number ''n'' of hydrogens have been replaced by ''n'' hydroxyls. In this case the number ''n'' is indicated by a multiplier prefix (mono-, di-, tri-, etc.), and the parent quinone's name is used instead of just "quinone" — as in tetrahydroxy-1,4-benzoquinone. File:Tetrahydroxy-1,4-benzoquinone-2D-skeletal.png, Tetrahydroxy-1,4-benzoquinone File:Juglone.png, 5-Hydroxy-1,4-naphthoquinone(Juglone) File:Alizarin chemical structure.png, 1, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxybenzoquinone
A hydroxybenzoquinone (formula: ) is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). In general, the term may mean any benzoquinone derivative where any number ''n'' of hydrogens have been replaced by ''n'' hydroxyls, so that the formula is . In this case the number ''n'' (which is between 1 and 4) is indicated by a multiplier prefix (mono-, di-, tri-, tetra-, penta-, or hexa-). The unqualified term "hydroxybenzoquinone" usually means a derivative of 1,4-benzoquinone. Other hydroxy- compounds can be derived from the other isomer, namely 1,2-benzoquinone or ''ortho''-benzoquinone. The IUPAC nomenclature uses dihydrobenzenedione instead of "benzoquinone", with the necessary prefixes to indicate the positions of the carbonyl oxygens (=O) — as in 2,3-dihydroxy-1a,4a-dihydrobenzene-1,4-dione (= 2,3-dihydroxy-1,4-benzoquinone). The hydroxybenzoquinones (in the particular or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Livin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydroxy-1,4-benzoquinone
Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-''p''-benzoquinone, tetrahydroxybenzoquinone, or tetrahydroxyquinone (THBQ, THQ), is an organic compound with formula . Its molecular structure consists of a cyclohexadiene ring with four hydroxyl groups and two ketone groups in opposite (''para'') positions. The compound gives a light red solution in water, and crystallizes as the glistening bluish-black (but non-conducting) dihydrate . The compound can be synthesized from glyoxal or from ''myo''-inositol, a natural compound widely present in plants. THBQ forms an adduct with 4,4′-bipyridine in a 2:3 ratio. Salts of THBQ Like most phenols, THBQ is acidic and easily loses the four hydrogen ions from the hydroxyl groups, yielding anions such as and . The latter is symmetric and aromatic, as the double bonds and negative charges are evenly distributed over the six CO groups. The calcium salt is the dark purple pigment produced from inositol by '' Chromohalobacter beij ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxynaphthoquinone
A hydroxynaphthoquinone (formula: ) is any of several organic compounds that can be viewed as derivatives of a naphthoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). In general, the term may mean any naphthoquinone derivative where any number ''n'' of hydrogens have been replaced by ''n'' hydroxyls, so that the formula is . In this case the number ''n'' (which is between 1 and 6) is indicated by a multiplier prefix (mono-, di-, tri-, tetra-, penta-, or hexa-). The unqualified term "hydroxynaphthoquinone" usually means a derivative of 1,4-naphthoquinone. Other hydroxy- compounds can be derived from other isomers of the latter, such as 1,2-naphthoquinone and 2,6-naphthoquinone. The IUPAC nomenclature uses dihydronaphthalenedione instead of "naphthoquinone", with the necessary prefixes to indicate the positions of the carbonyl oxygens (=O) — as in 5,8-dihydroxy-1a,8a-dihydronaphthalene-1,4-dione (= 5,8-dihydroxy-1,4-naphthoquinone). The hydroxyn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''para'' position. It is a white granular solid. Substituted derivatives of this parent compound are also referred to as hydroquinones. The name "hydroquinone" was coined by Friedrich Wöhler in 1843. Production Hydroquinone is produced industrially in two main ways.Phillip M. Hudnall "Hydroquinone" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. 2005 Wiley-VCH, Weinheim. . * The most widely used route is similar to the cumene process in reaction mechanism and involves the dialkylation of benzene with propene to give 1,4-diisopropylbenzene. This compound reacts with air to afford the bis(hydroperoxide), which is structurally similar to cumene hydroperoxide and rearranges in acid to give acetone and hydroquinone. * A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research.Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells and between cells, Karp (2009), p. 2. in turn relating greatly to the understanding of tissues and organs, as well as organism structure and function. Miller (2012). p. 62. Biochemistry is closely related to molecular biology, which is the study of the molecular mechanisms of biological phenom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully cov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered reta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medicinal Drug
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in multiple ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the order of a physician, physician assistant, or qualified nurse) from over-the-counter drugs (those that consumers can order for themselves). Another key distinction is between traditional small molecule drugs, usually derived from chemical synthesis, and biopharmaceuticals, which include recombinant proteins, vaccines, blood products used therapeutically (such as IVIG), gene therapy, monoclonal antibodies and cell therapy (for instance, stem cell therapies). Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prefix
A prefix is an affix which is placed before the stem of a word. Adding it to the beginning of one word changes it into another word. For example, when the prefix ''un-'' is added to the word ''happy'', it creates the word ''unhappy''. Particularly in the study of languages, a prefix is also called a preformative, because it alters the form of the words to which it is affixed. Prefixes, like other affixes, can be either inflectional, creating a new form of the word with the same basic meaning and same lexical category (but playing a different role in the sentence), or derivational, creating a new word with a new semantic meaning and sometimes also a different lexical category. Prefixes, like all other affixes, are usually bound morphemes. In English, there are no inflectional prefixes; English uses suffixes instead for that purpose. The word ''prefix'' is itself made up of the stem ''fix'' (meaning "attach", in this case), and the prefix ''pre-'' (meaning "before"), bot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoquinone
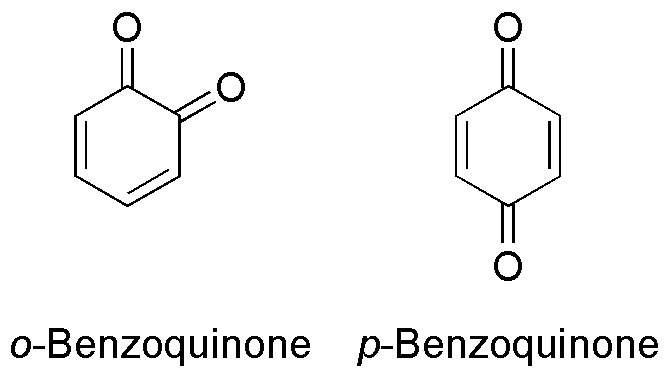
Benzoquinone (C6H4O2) is a quinone with a single benzene ring. There are 2 (out of 3 hypothetical) benzoquinones: * 1,4-Benzoquinone, most commonly, right image (also ''para''-benzoquinone, ''p''-benzoquinone, ''para''-quinone, or just quinone) * 1,2-Benzoquinone, less commonly, left image (also ''ortho''-benzoquinone, ''o''-benzoquinone, ''ortho''-quinone) *1,3-benzoquinone "does not exist, because its structure would be nonplanar and highly strained", though derivatives are known. An alkylated ''p''-benzoquinone has been found in the rhizomes of '' Iris kemaonensis''. See also * Arene substitution pattern Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon. ''Ortho'', ''meta'', and ''para'' substitution * I ... References {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula . Isomers include various quinone derivatives. The term anthraquinone however refers to the isomer, 9,10-anthraquinone (IUPAC: 9,10-dioxoanthracene) wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite. Synthesis There are several current industrial methods to produce 9,10-anthraquinone: # The oxidation of anthracene. Chromium(VI) is the typical oxidant. # The Friedel-Crafts reaction of benzene and phthalic anhydride in presence of AlCl3. o-Benzoylbenzoic acid is an intermediate. This reaction is useful for produci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |