|
Freeze-distilled
Fractional freezing is a process used in process engineering and chemistry to Separation process, separate substances with different melting points. It can be done by partial melting of a solid, for example in zone melting, zone refining of silicon or metals, or by partial crystallization of a liquid, as in freeze distillation, also called normal freezing or progressive freezing. The initial sample is thus fractionation, fractionated (separation process, separated into fraction (chemistry), fractions). Partial crystallization can also be achieved by adding a dilute solvent to the mixture, and cooling and concentrating the mixture by evaporating the solvent, a process called solution crystallization. Fractional freezing is generally used to produce ultra-pure solids, or to concentrate heat-sensitive liquids. Freeze distillation Freeze distillation is actually a condensation, not distillation per se. It removes frozen solid, separating a dissolved material from the liquid left be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Wine Grapes
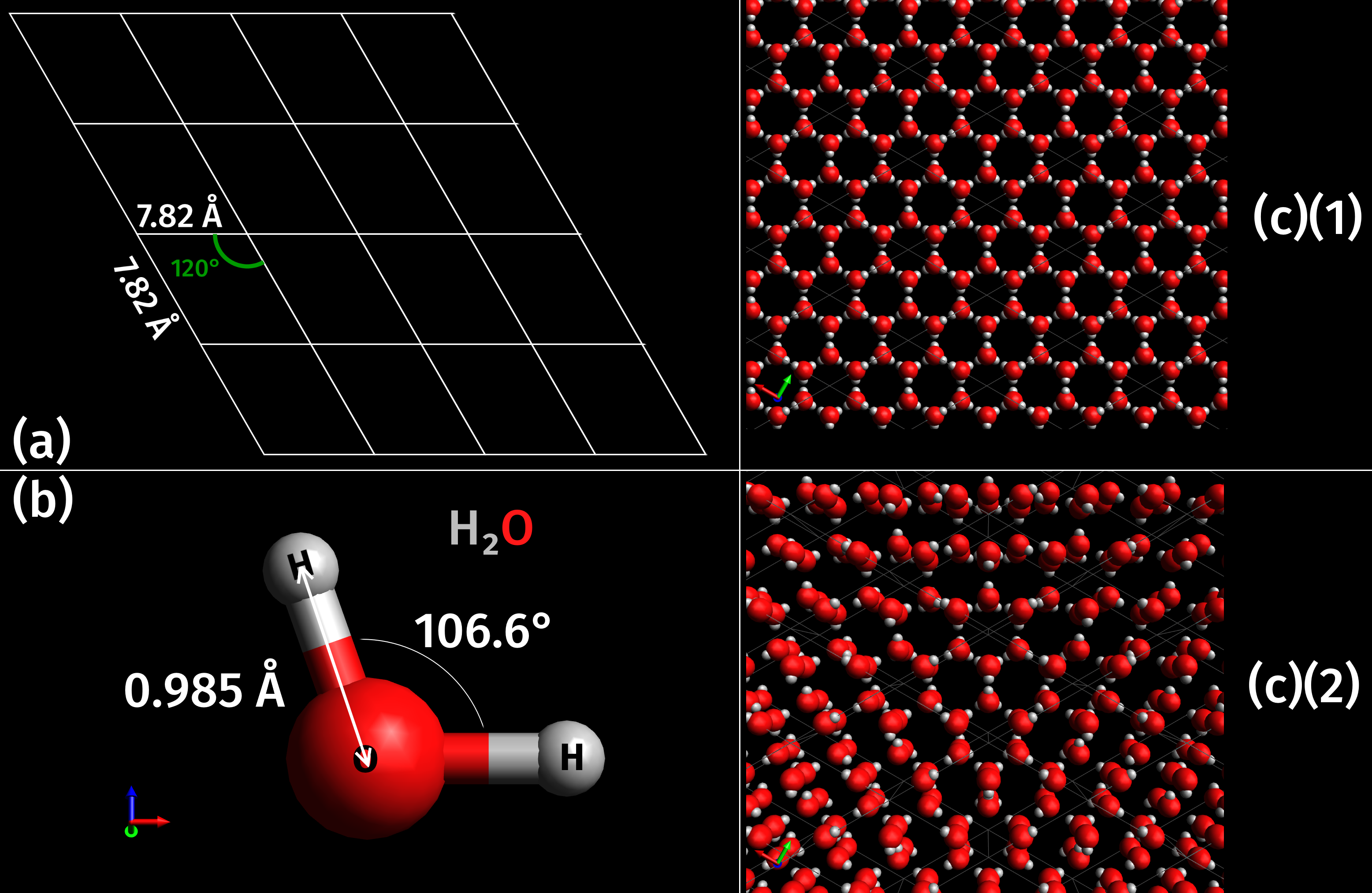
Ice is water that is freezing, frozen into a solid state, typically forming at or below temperatures of 0 °Celsius, C, 32 °Fahrenheit, F, or 273.15 Kelvin, K. It occurs naturally on Earth, on other planets, in Oort cloud objects, and as interstellar ice. As a naturally occurring crystalline inorganic solid with an ordered structure, ice is considered to be a mineral. Depending on the presence of Impurity, impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less Opacity (optics), opaque bluish-white color. Virtually all of the ice on Earth is of a Hexagonal crystal system, hexagonal Crystal structure, crystalline structure denoted as ''ice Ih'' (spoken as "ice one h"). Depending on temperature and pressure, at least nineteen phases of ice, phases (Sphere packing, packing geometries) can exist. The most common phase transition to ice Ih occurs when liquid water is cooled below (, ) at standard atmospheric pressure. When water is coo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Beer
Ice beer is a beer that has undergone some degree of freezing during production. These beers generally have a higher alcohol content, and lower price relative to it. The process of "icing" beer involves lowering the temperature until ice crystals form. Since ethanol has a much lower freezing point (-114 °C; -173.2 °F) than water (0 °C; 32 °F), when the ice is removed the alcohol concentration of the beer increases. The process is known as fractional freezing or freeze distillation. History Ice beer was developed by brewing a strong, dark lager, then freezing the beer and removing some of the ice. This concentrates the aroma and taste of the beer, and also raises the alcoholic strength of the finished beer. This produces a beer with 12 to 15 per cent alcohol. In North America, water would be added to lower the alcohol level. Eisbock was introduced to Canada in 1989 by the microbrewery Niagara Falls Brewing Company. The brewers started with a strong dark l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aventinus Weizen-Eisbock
Aventinus may refer to: Places: * Aventinus, Latin name of Abensberg, Germany * Aventine Hill, named after Aventinus, king of Alba and Latium Persons: * Aventinus (mythology) Aventinus was a son of Hercules and the priestess Rhea mentioned in Virgil's ''Aeneid'', Book vii. 656, as an ally of Mezentius and enemy of Aeneas (Dryden's translation): Maurus Servius Honoratus, Servius This passage speaks of an Aventinus, ..., son of Hercules and Rhea * Aventinus of Alba Longa, descendant of Aeneas, king of the Latins (future Rome site) * Saint Aventinus (d. c 537), disciple of St. Loup * Aventinus of Tours (d. 1180), hermit and saint * Johannes Aventinus, Bavarian historian and philologist Others: * Aventinus (beer), a wheat doppelbock brewed by G. Schneider & Sohn, in Bavaria, Germany See also * Aventine {{disambig, surname, geo Latin-language surnames ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polynyas
A polynya () is an area of open water surrounded by sea ice. It is now used as a geographical term for an area of unfrozen seawater within otherwise contiguous pack ice or fast ice. It is a loanword from the Russian (), which refers to a natural ice hole and was adopted in the 19th century by polar explorers to describe navigable portions of the sea. There are two main types of polynyas: coastal polynyas, which can be found year-round near the Antarctic and Arctic coasts and are mainly created by strong winds pushing the ice away from the coast, and mid-sea or open-ocean polynyas, which may be found more sporadically in the middle of ice pack in certain locations, especially around Antarctica. These locations are generally preconditioned by certain oceanic dynamics. One of the most famous mid-sea polynyas is the Weddell Polynya, also known as the Maud Rise Polynya, which occurs in the Lazarev Sea over the Maud Rise seamount. It was first spotted in September 1973, persis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brine Rejection
Brine rejection is a process that occurs when salty water freezes. The salts do not fit in the crystal structure of water ice, so the salt is expelled. Since the oceans are salty, this process is important in nature. Salt rejected by the forming sea ice drains into the surrounding seawater, creating saltier, denser brine. The denser brine sinks, influencing ocean circulation. Formation As water reaches the temperature where it begins to crystallize and form ice, salt ions are rejected from the lattices within the ice and are either forced out into the surrounding water, or trapped among the ice crystals in pockets called brine cells. Generally, sea ice has a salinity ranging from 0 psu at the surface to 4 psu at the base. The faster that this freezing process occurs, the more brine cells are left in the ice. Once the ice reaches a critical thickness, roughly 15 cm, the concentration of salt ions in the liquid around the ice begins to increase, as leftover brine is rejecte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilibrium, equilibrium. The melting point of a substance depends on pressure and is usually specified at a Standard temperature and pressure, standard pressure such as 1 Atmosphere (unit), atmosphere or 100 Pascal (unit), kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to Supercooling, supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the #Melting point measurements, melting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs as the mineral halite. In its edible form, it is commonly used as a condiment and curing (food preservation), food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further Chemical synthesis, chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather. Uses In addition to the many familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 data) include chemicals and de-icing.Westphal, Gisbert ''et al.'' (2002) "Sodium Chloride" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim . Chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desalination
Desalination is a process that removes mineral components from saline water. More generally, desalination is the removal of salts and minerals from a substance. One example is Soil salinity control, soil desalination. This is important for agriculture. It is possible to desalinate saltwater, especially Seawater, sea water, to produce water for human consumption or irrigation. The by-product of the desalination process is brine. Many seagoing ships and submarines use desalination. Modern interest in desalination mostly focuses on cost-effective provision of fresh water for human use. Along with recycled wastewater, it is one of the few water resources independent of rainfall. Due to its energy consumption, desalinating sea water is generally more costly than fresh water from surface water or groundwater, Reclaimed water, water recycling and water conservation; however, these alternatives are not always available and depletion of reserves is a critical problem worldwide. Desalinati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling. On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate unt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentrate
A concentrate is a form of Chemical substance, substance that has had the majority of its diluting agent or diluent (in the case of a liquid: the solvent) removed, such that the substance becomes the majority of the composition. Typically, this will be the removal of water from a Solution (chemistry), solution or suspension (chemistry), suspension, such as the removal of water from fruit juice. Food Juice concentrate A juice concentrate is the result of removing water from fruit or vegetable juice. In juice manufacturing from concentrate, numerous procedures are required under government regulation to ensure food safety. A process of concentrating orange juice was patented in 1948. It was originally developed to provide World War II troops with a reliable source of vitamin C. Soft drink concentrate Most Soft drink, sodas and soft drinks are produced as highly concentrated syrups and later diluted with carbonated water directly before consumption or bottling. Such concentra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fruit Juice
Juice is a drink made from the extraction or pressing of the natural liquid contained in fruit and vegetables. It can also refer to liquids that are flavored with concentrate or other biological food sources, such as meat or seafood, such as clam juice. Juice is commonly consumed as a beverage or used as an ingredient or flavoring in foods or other beverages, such as smoothies. Juice emerged as a popular beverage choice after the development of pasteurization methods enabled its preservation without using fermentation (which is used in wine production). The largest fruit juice consumers are New Zealand (nearly a cup, or 8 ounces, each day) and Colombia (more than three quarters of a cup each day). Fruit juice consumption on average increases with a country's income level. Etymology The word "juice" comes from Old French in about 1300; it developed from the Old French words "''jus, juis, jouis''", which mean "liquid obtained by boiling herbs". The Old French ''jus'' "j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azeotrope
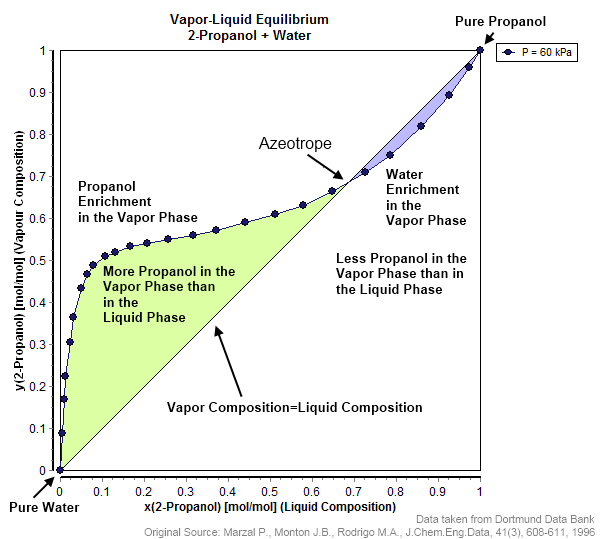
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Knowing an azeotrope's behavior is important for distillation. Each azeotrope has a characteristic boiling point. The boiling point of an azeotrope is either less than the boiling point temperatures of any of its constituents (a positive azeotrope), or greater than the boiling point of any of its constituents (a negative azeotrope). For both positive and negative azeotropes, it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. For technical applications, the pressure-temperature-composition behavior of a mixture is the most important, but other important ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |