|
Electrocatalyst
An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells. Background and theory An electrocatalyst lowers the activation energy required for an electrochemical reaction. Some electrocatalysts change the potential at which oxidation and reduction processes occur. In other cases, an electrocatalyst can impart selectivity by favoring specific chemical interaction at an electrode surface. Given that electrochemical reactions occur when electrons are passed from one chemical sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolysis Of Water
Electrolysis of water is using electricity to Water splitting, split water into oxygen () and hydrogen () gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C. Water electrolysis requires a minimum potential difference of 1.23 volts, although at that voltage external heat is also required. Typically 1.5 volts is required. Electrolysis is rare in industrial applications since hydrogen can be produced less expensively from fossil fuels. Most of the time, hydrogen is made by splitting methane (CH4) into carbon dioxide (CO2) and hydrogen (H2) via steam reforming. This is a carbon-intensive process that means for every kilogram of “grey” hydrogen produced, approximatel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
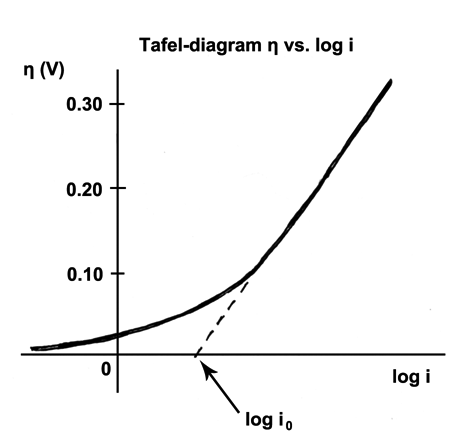
Tafel Equation
The Tafel equation is an equation in electrochemical kinetics relating the rate of an Electrochemistry, electrochemical reaction to the overpotential. The Tafel equation was first deduced experimentally and was later shown to have a theoretical justification. The equation is named after Swiss chemist Julius Tafel.It describes how the electrical current through an electrode depends on the voltage difference between the electrode and the bulk electrolyte for a simple, unimolecular redox reaction. : Ox + n e^- \leftrightarrows Red Where an electrochemical reaction occurs in two half reactions on separate electrodes, the Tafel equation is applied to each electrode separately. On a single electrode the Tafel equation can be stated as: where * the plus sign under the exponent refers to an anodic reaction, and a minus sign to a cathodic reaction, * \eta : overpotential, [V] * A : "Butler–Volmer equation#The limiting cases, Tafel slope", [V] * i : current density, [A/m2] * i_0 : "exch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most battery (electricity), batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied. The first fuel cells were invented by Sir William Robert Grove, William Grove in 1838. The first commercial use of fuel cells came almost a century later following the invention of the hydrogen–oxygen fuel cell by Francis Thomas Bacon in 1932. The alkaline fuel cell, also known as the Bacon fuel cell after its inventor, has been used in NASA space programs since the mid-1960s to generate power for sate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically conducting phase (typically an external electrical circuit, but not necessarily, as in electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic species in a solution). When a chemical reaction is driven by an electrical potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the aforementioned electronically conducting circuit. This phenomenon is what distinguishes an electrochemical reaction from a conventional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Evolution Reaction
Hydrogen evolution reaction (HER) is a chemical reaction that yields H2. The conversion of protons to H2 requires reducing equivalents and usually a catalyst. In nature, HER is catalyzed by hydrogenase enzymes which rely on iron- and nickel-based catalysts. Commercial electrolyzers typically employ supported nickel-based catalysts. HER in electrolysis HER is a key reaction which occurs in the electrolysis of water for the production of hydrogen for both industrial energy applications, as well as small-scale laboratory research. Due to the abundance of water on Earth, hydrogen production poses a potentially scalable process for fuel generation. This is an alternative to steam methane reforming for hydrogen production, which has significant greenhouse gas emissions, and as such scientists are looking to improve and scale up electrolysis processes that have fewer emissions. Electrolysis mechanism In acidic conditions, the hydrogen evolution reaction follows the formula: : In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Reaction Mechanism
In electrochemistry, an electrochemical reaction mechanism is the step-by-step sequence of elementary steps, involving at least one outer-sphere electron transfer, by which an overall electrochemical reaction occurs. Overview Elementary steps like proton coupled electron transfer and the movement of electrons between an electrode and substrate are special to electrochemical processes. Electrochemical mechanisms are important to all redox chemistry including corrosion, redox active photochemistry including photosynthesis, other biological systems often involving electron transport chains and other forms of homogeneous and heterogeneous electron transfer. Such reactions are most often studied with standard three electrode techniques such as cyclic voltammetry(CV), chronoamperometry, and bulk electrolysis as well as more complex experiments involving rotating disk electrodes and rotating ring-disk electrodes. In the case of photoinduced electron transfer the use of ti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety of materials (chemicals) depending on the type of cell. An electrode may be called either a cathode or anode according to the direction of the electric current, unrelated to the potential difference between electrodes. Michael Faraday coined the term "" in 1833; the word recalls the Greek ἤλεκτρον (, "amber") and ὁδός (, "path, way"). The electrophore, invented by Johan Wilcke in 1762, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Types Of Electrocatalysts
Type may refer to: Science and technology Computing * Typing, producing text via a keyboard, typewriter, etc. * Data type, collection of values used for computations. * File type * TYPE (DOS command), a command to display contents of a file. * Type (Unix), a command in POSIX shells that gives information about commands. * Type safety, the extent to which a programming language discourages or prevents type errors. * Type system, defines a programming language's response to data types. Mathematics * Type (model theory) * Type theory, basis for the study of type systems * Arity or type, the number of operands a function takes * Type, any proposition or set in the intuitionistic type theory * Type, of an entire function ** Exponential type Biology * Type (biology), which fixes a scientific name to a taxon * Dog type, categorization by use or function of domestic dogs Lettering * Type is a design concept for lettering used in typography which helped bring about modern textual printi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |