|
Dodecahedrane
Dodecahedrane is a chemical compound, a hydrocarbon with formula , whose carbon atoms are arranged as the vertices (corners) of a regular dodecahedron. Each carbon is bound to three neighbouring carbon atoms and to a hydrogen atom. This compound is one of the three possible Platonic hydrocarbons, the other two being cubane and tetrahedrane. Dodecahedrane does not occur in nature and has no significant uses. It was synthesized by Leo Paquette in 1982, primarily for the "aesthetically pleasing symmetry of the dodecahedral framework". For many years, dodecahedrane was the simplest real carbon-based molecule with full icosahedral symmetry. Buckminsterfullerene (), discovered in 1985, also has the same symmetry, but has three times as many carbons and 50% more atoms overall. The synthesis of the C20 fullerene in 2000, from brominated dodecahedrane, may have demoted to second place. Structure The angle between the C-C bonds in each carbon atom is 108°, which is the angle betwe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leo Paquette
Leo Armand Paquette ( – January 21, 2019) was an American organic chemist. Biography Paquette was born on July 15, 1934, in Worcester, Massachusetts. He received a Bachelor of Science (B.S.) in 1956 from the College of the Holy Cross and a Ph.D. in organic chemistry from the Massachusetts Institute of Technology in 1959, under the supervision of Norman Allan Nelson. After serving as a research associate at the Upjohn Company from 1959 to 1963, he joined the faculty of Ohio State University (OSU) where he mentored approximately 150 doctoral students, 300 postdoctoral associates, and countless masters students and undergraduates. Paquette was promoted to full professor at OSU in 1969 and was named Distinguished University Professor in 1987. He was elected a member of the National Academy of Sciences in 1984, and was the founding editor of the '' Electronic Encyclopedia of Reagents for Organic Synthesis (e-EROS). '' Paquette is best known for achieving the first total synthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platonic Hydrocarbons
In organic chemistry, a Platonic hydrocarbon is a hydrocarbon whose structure matches one of the five Platonic solids, with carbon atoms replacing its vertices, carbon–carbon bonds replacing its edges, and hydrogen atoms as needed. Not all Platonic solids have molecular hydrocarbon counterparts; those that do are the tetrahedron ( tetrahedrane), the cube (cubane), and the dodecahedron ( dodecahedrane). The possibility and existence of each platonic hydrocarbon is affected by the number of bonds to each carbon vertex and the angle strain between the bonds at each vertex. Tetrahedrane Tetrahedrane (C4H4) is a hypothetical compound. It has not yet been synthesized without substituents, but it is predicted to be kinetically stable in spite of its angle strain. Some stable derivatives, including tetra( ''tert''-butyl)tetrahedrane and tetra(trimethylsilyl)tetrahedrane, have been produced. Cubane Cubane (C8H8) has been synthesized. Although it has high angle strain, cubane is k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Symmetry
In chemistry, molecular symmetry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain many of a molecule's chemical property, chemical properties, such as whether or not it has a molecular dipole moment, dipole moment, as well as its allowed spectroscopy, spectroscopic transitions. To do this it is necessary to use group theory. This involves classifying the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Symmetry is useful in the study of molecular orbitals, with applications to the Hückel method, to ligand field theory, and to the Woodward–Hoffmann rules. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry discuss symmetry. Another framework on a larger scale is the use of crystal sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Icosahedral Symmetry
In mathematics, and especially in geometry, an object has icosahedral symmetry if it has the same symmetries as a regular icosahedron. Examples of other polyhedra with icosahedral symmetry include the regular dodecahedron (the dual polyhedron, dual of the icosahedron) and the rhombic triacontahedron. Every polyhedron with icosahedral symmetry has 60 Rotational symmetry, rotational (or orientation-preserving) symmetries and 60 orientation-reversing symmetries (that combine a rotation and a Reflection symmetry, reflection), for a total symmetry order of 120. The full symmetry group is the Coxeter group of type . It may be represented by Coxeter notation and Coxeter diagram . The set of rotational symmetries forms a subgroup that is isomorphic to the alternating group on 5 letters. As point group Apart from the two infinite series of prismatic and antiprismatic symmetry, rotational icosahedral symmetry or chiral icosahedral symmetry of chiral objects and full icosahedra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
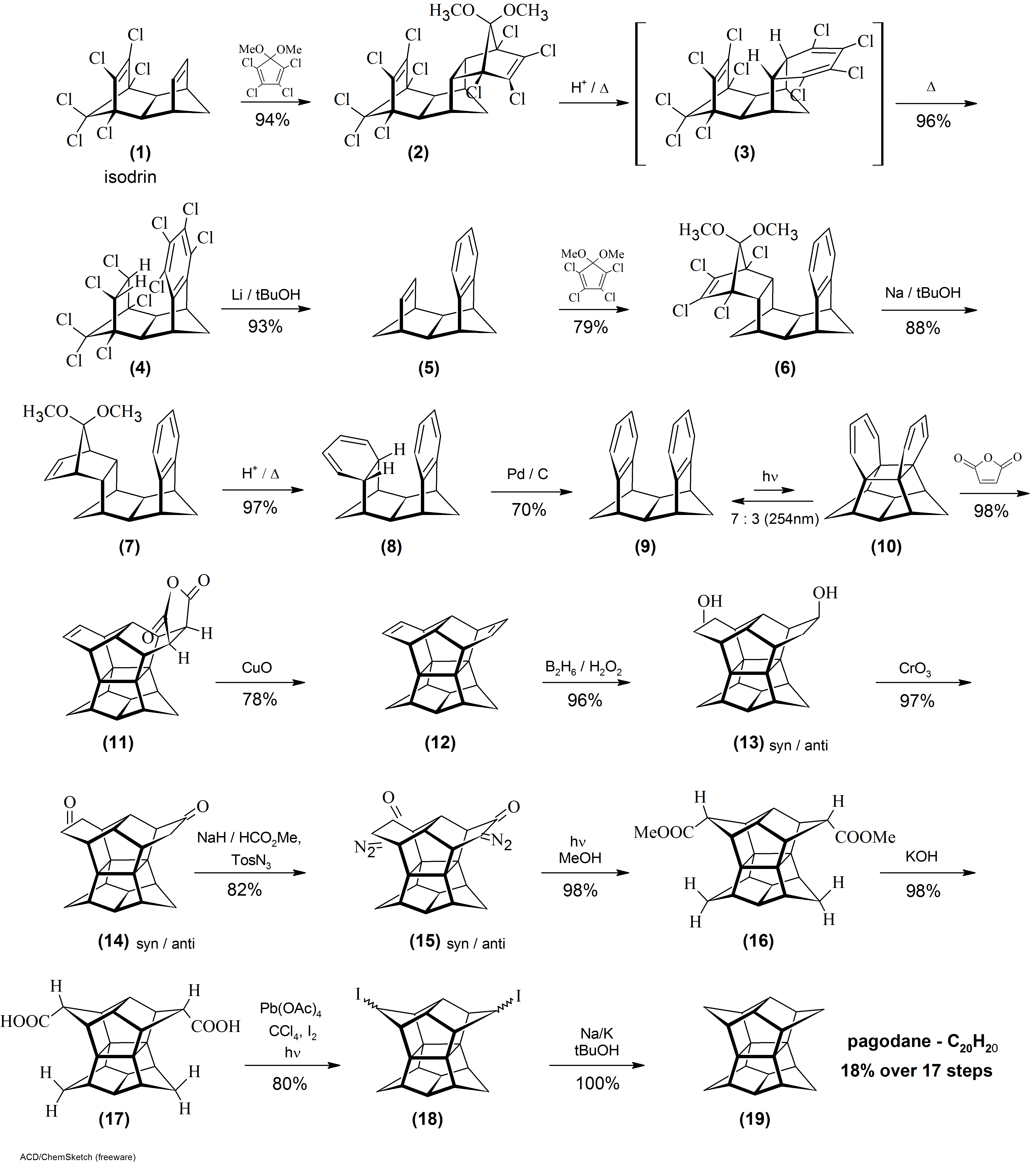
Pagodane
Pagodane is an organic compound with formula whose carbon skeleton was said to resemble a pagoda, hence the name. It is a polycyclic hydrocarbon whose molecule has the ''D''2''h'' point symmetry group. The compound is a highly crystalline solid that melts at 243 °C, is barely soluble in most organic solvents and moderately soluble in benzene and chloroform. It sublimes at low pressure. The name pagodane is used more generally for any member of a family of compounds whose molecular skeletons have the same 16-carbon central cage as the basic compound. Each member can be seen as the result of connecting eight atoms of this cage in pairs by four alkane chains. The general member is denoted 'm''.''n''.''p''.''q''agodane where ''m'', ''n'', ''p'' and ''q'' are the number of carbons of those four chains. The general formula is then where ''s''= ''m''+''n''+''p''+''q''. In particular, the basic compound has those carbons connected by four methylene bridges (''m''=''n''=''p''='' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proc Natl Acad Sci U S A
''Proceedings of the National Academy of Sciences of the United States of America'' (often abbreviated ''PNAS'' or ''PNAS USA'') is a peer-reviewed multidisciplinary scientific journal. It is the official journal of the National Academy of Sciences, published since 1915, and publishes original research, scientific reviews, commentaries, and letters. According to ''Journal Citation Reports'', the journal has a 2022 impact factor of 9.4. ''PNAS'' is the second most cited scientific journal, with more than 1.9 million cumulative citations from 2008 to 2018. In the past, ''PNAS'' has been described variously as "prestigious", "sedate", "renowned" and "high impact". ''PNAS'' is a delayed open-access journal, with an embargo period of six months that can be bypassed for an author fee ( hybrid open access). Since September 2017, open access articles are published under a Creative Commons license. Since January 2019, ''PNAS'' has been online-only, although print issues are available ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eclipsed Conformation
In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X–A–B–Y is 0°. Such a conformation can exist in any open chain, single chemical bond connecting two sp3- hybridised atoms, and it is normally a conformational energy maximum. This maximum is often explained by steric hindrance, but its origins sometimes actually lie in hyperconjugation (as when the eclipsing interaction is of two hydrogen atoms). In the example of ethane, two methyl groups are connected with a carbon-carbon sigma bond, just as one might connect two Lego pieces through a single "stud" and "tube". With this image in mind, if the methyl groups are rotated around the bond, they will remain connected; however, the shape will change. This leads to multiple possible three-dimensional arrangements, known as conformations, conformational isomers (conformers), or sometimes rotational isome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some Stress (mechanics), stress which raises its internal energy in comparison to a strain-free reference Chemical compound, compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional amount of internal energy which an unstrained molecule does not. This extra internal energy, or strain energy, can be likened to a compression (physics), compressed spring (device), spring.Anslyn and Dougherty, ''Modern Physical Organic Chemistry'', University Science Books, 2006, Much like a compressed spring must be held in place to prevent release of its potential energy, a molecule can be held in an energetically unfavorable conformation by the Chemical bond, bonds within that molecule. Without the bonds holding the conformation in place, the strain energy would be released. Summary Thermodynamics The Chemical equilibrium, equilibrium of two c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angle Strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated. Ring strain results from a combination of angle strain, conformational strain or Pitzer strain (torsional eclipsing interactions), and transannular strain, also known as van der Waals strain or Prelog strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane. Ring strain energy can be attributed to the energy required for the distortion of bond and bond angles in order to close a ring. Ring strain energy is believed to be the cause of accelerated rates in altering ring reactions. Its interactions with traditional bond energies chan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C20 Fullerene
C, or c, is the third letter of the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''cee'' (pronounced ), plural ''cees''. History "C" comes from the same letter as "G". The Semites named it gimel. The sign is possibly adapted from an Egyptian hieroglyph for a staff sling, which may have been the meaning of the name ''gimel''. Another possibility is that it depicted a camel, the Semitic name for which was ''gamal''. Barry B. Powell, a specialist in the history of writing, states "It is hard to imagine how gimel = "camel" can be derived from the picture of a camel (it may show his hump, or his head and neck!)". In the Etruscan language, plosive consonants had no contrastive voicing, so the Greek ' Γ' (Gamma) was adopted into the Etruscan alphabet to represent . Already in the Western Greek alphabet, Gamma first took a '' form in Early Etruscan, then '' in Classical Etru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regular Tetrahedron
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tetrahedron is the simplest of all the ordinary convex polytope, convex polyhedra. The tetrahedron is the three-dimensional case of the more general concept of a Euclidean geometry, Euclidean simplex, and may thus also be called a 3-simplex. The tetrahedron is one kind of pyramid (geometry), pyramid, which is a polyhedron with a flat polygon base and triangular faces connecting the base to a common point. In the case of a tetrahedron, the base is a triangle (any of the four faces can be considered the base), so a tetrahedron is also known as a "triangular pyramid". Like all convex polyhedra, a tetrahedron can be folded from a single sheet of paper. It has two such net (polyhedron), nets. For any tetrahedron there exists a sphere (called th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |