|
DWSIM Icon
__NOTOC__ DWSIM is an Open-source software, open-source CAPE-OPEN Interface Standard, CAPE-OPEN compliant chemical Process simulation, process simulator for Windows, Linux and macOS. DWSIM is built on top of the .NET Framework, Microsoft .NET and Mono (software), Mono Platforms and features a graphical user interface (GUI), advanced thermodynamics calculations, reactions support and petroleum characterization / hypothetical component generation tools. DWSIM is able to simulate steady-state, vapor–liquid, vapor–liquid-liquid, solid–liquid and aqueous electrolyte equilibrium processes with the following Thermodynamic Models and Unit Operations: * thermodynamics, Thermodynamic modelsCoolProp Peng–Robinson equation of state, Peng–Robinson–Stryjek–Vera equation of state, Peng–Robinson-Strÿjek-Vera (PRSV2), Equation of state, Soave–Redlich–Kwong, Lee-Kesler method, Lee-Kesler, Lee-Kesler-Plöcker, UNIFAC, UNIFAC(-LL), Modified UNIFAC (Dortmund), Modified UNIFAC (NIST ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Open-source Software
Open-source software (OSS) is Software, computer software that is released under a Open-source license, license in which the copyright holder grants users the rights to use, study, change, and Software distribution, distribute the software and its source code to anyone and for any purpose. Open-source software may be developed in a collaborative, public manner. Open-source software is a prominent example of open collaboration, meaning any capable user is able to online collaboration, participate online in development, making the number of possible contributors indefinite. The ability to examine the code facilitates public trust in the software. Open-source software development can bring in diverse perspectives beyond those of a single company. A 2024 estimate of the value of open-source software to firms is $8.8 trillion, as firms would need to spend 3.5 times the amount they currently do without the use of open source software. Open-source code can be used for studying and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unit Operation
In chemical engineering and related fields, a unit operation is a basic step in a process. Unit operations involve a physical change or chemical transformation such as separation, crystallization, evaporation, filtration, polymerization, isomerization, and other reactions. For example, in milk processing, the following unit operations are involved: homogenization, pasteurization, and packaging. These unit operations are connected to create the overall process. A process may require many unit operations to obtain the desired product from the starting materials, or feedstocks. History Historically, the different chemical industries were regarded as different industrial processes and with different principles. Arthur Dehon Little developed the concept of "unit operations" to explain industrial chemistry processes in 1916. In 1923, William H. Walker, Warren K. Lewis and William H. McAdams wrote the book ''The Principles of Chemical Engineering'' and explained that the variety of c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
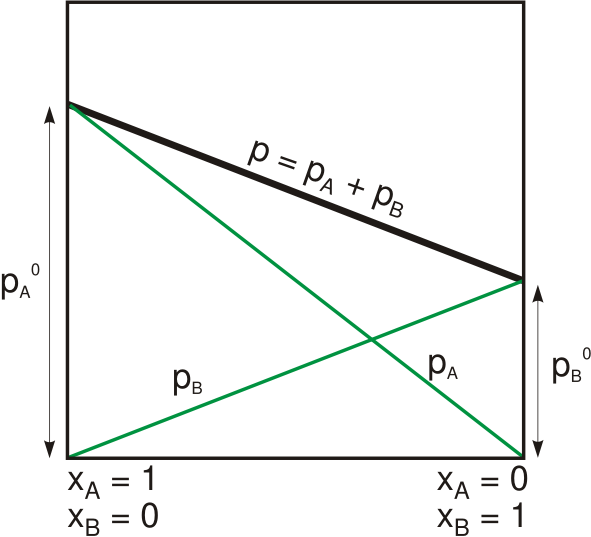
Raoult's Law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is equal to the vapor pressure of the pure component (liquid or solid) multiplied by its mole fraction in the mixture. In consequence, the relative lowering of vapor pressure of a dilute solution of nonvolatile solute is equal to the mole fraction of solute in the solution. Mathematically, Raoult's law for a single component in an ideal solution is stated as : p_i = p_i^\star x_i where p_i is the partial pressure of the component i in the gaseous mixture above the solution, p_i^\star is the equilibrium vapor pressure of the pure component i, and x_i is the mole fraction of the component i in the liquid or solid solution. Where two volatile liquids A and B are mixed with each other to form a solution, the vapor phase consists of both compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
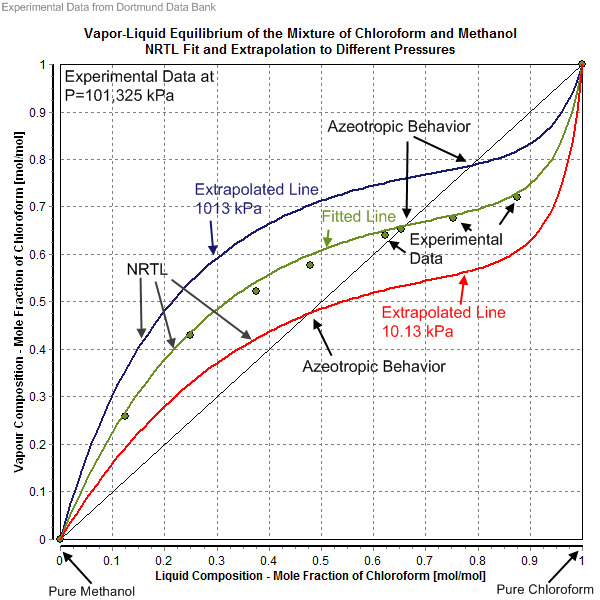
Non-random Two-liquid Model
The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model introduced by Renon and John Prausnitz, Prausnitz in 1968 that correlates the activity coefficients \gamma_i of a compound with its mole fractions x_i in the liquid phase concerned. It is frequently applied in the field of chemical engineering to calculate phase equilibria. The concept of NRTL is based on the hypothesis of Wilson, who stated that the local concentration around a molecule in most mixtures is different from the bulk concentration. This difference is due to a difference between the interaction energy of the central molecule with the molecules of its own kind U_ and that with the molecules of the other kind U_. The energy difference also introduces a non-randomness at the local molecular level. The NRTL model belongs to the so-called local-composition models. Other models of this type are the Wilson model, the UNIQUAC model, and the group contribution model UNIFAC. These local-com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
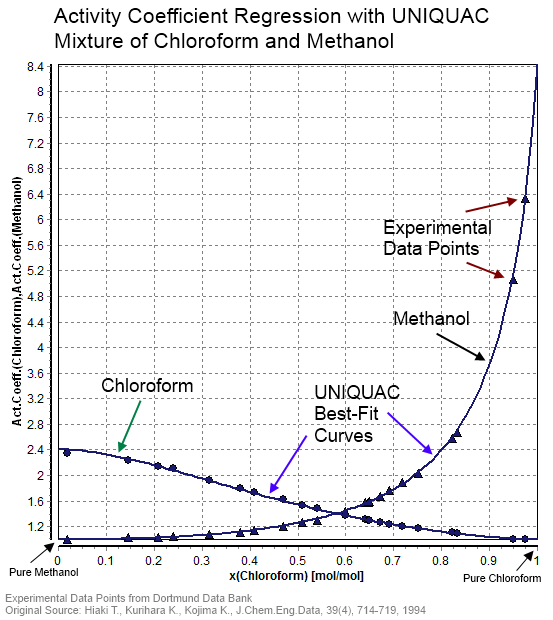
UNIQUAC
In statistical thermodynamics, UNIQUAC (a portmanteau of universal quasichemical) is an activity coefficient model used in description of phase equilibria. The model is a so-called lattice model and has been derived from a first order approximation of interacting molecule surfaces. The model is, however, not fully thermodynamically consistent due to its two- liquid mixture approach. In this approach the local concentration around one central molecule is assumed to be independent from the local composition around another type of molecule. The UNIQUAC model can be considered a second generation activity coefficient because its expression for the excess Gibbs energy consists of an entropy term in addition to an enthalpy term. Earlier activity coefficient models such as the Wilson equation and the non-random two-liquid model (NRTL model) only consist of enthalpy terms. Today the UNIQUAC model is frequently applied in the description of phase equilibria (i.e. liquid–solid, liqui ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UNIFAC
In statistical thermodynamics, the UNIFAC method ( UNIQUAC Functional-group Activity Coefficients)Aage Fredenslund, Russell L. Jones and John M. Prausnitz, "Group-Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures", ''AIChE Journal'', vol. 21 (1975), p. 1086 is a semi-empirical system for the prediction of non-electrolyte activity in non-ideal mixtures. UNIFAC uses the functional groups present on the molecules that make up the liquid mixture to calculate activity coefficients. By using interactions for each of the functional groups present on the molecules, as well as some binary interaction coefficients, the activity of each of the solutions can be calculated. This information can be used to obtain information on liquid equilibria, which is useful in many thermodynamic calculations, such as chemical reactor design, and distillation calculations. The UNIFAC model was first published in 1975 by Fredenslund, Jones and John Prausnitz, a group of chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equation Of State
In physics and chemistry, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most modern equations of state are formulated in the Helmholtz free energy. Equations of state are useful in describing the properties of pure substances and mixtures in liquids, gases, and solid states as well as the state of matter in the interior of stars. Though there are many equations of state, none accurately predicts properties of substances under all conditions. The quest for a universal equation of state has spanned three centuries. Overview At present, there is no single equation of state that accurately predicts the properties of all substances under all conditions. An example of an equation of state correlates densities of gases and liquids to temperatures and pressures, known as the ideal gas law, which is roughly accurate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peng–Robinson–Stryjek–Vera Equation Of State
In physics and chemistry, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most modern equations of state are formulated in the Helmholtz free energy. Equations of state are useful in describing the properties of pure substances and mixtures in liquids, gases, and solid states as well as the state of matter in the interior of stars. Though there are many equations of state, none accurately predicts properties of substances under all conditions. The quest for a universal equation of state has spanned three centuries. Overview At present, there is no single equation of state that accurately predicts the properties of all substances under all conditions. An example of an equation of state correlates densities of gases and liquids to temperatures and pressures, known as the ideal gas law, which is roughly accurate fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peng–Robinson Equation Of State
Cubic equations of state are a specific class of thermodynamic models for modeling the pressure of a gas as a function of temperature and density and which can be rewritten as a cubic function of the molar volume. Equations of state are generally applied in the fields of physical chemistry and chemical engineering, particularly in the modeling of vapor–liquid equilibrium and chemical engineering process design. Van der Waals equation of state The van der Waals equation of state may be written as : \left(p + \frac\right)\left(V_\text - b\right) = RT where T is the absolute temperature, p is the pressure, V_\text is the molar volume and R is the universal gas constant. Note that V_\text = V / n, where V is the volume, and n=N/N_\text, where n is the number of moles, N is the number of particles, and N_\text is the Avogadro constant. These definitions apply to all equations of state below as well. Proposed in 1873, the van der Waals equation of state was one of the first to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantity, physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the thermodynamic efficiency, efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |