UNIQUAC on:
[Wikipedia]
[Google]
[Amazon]
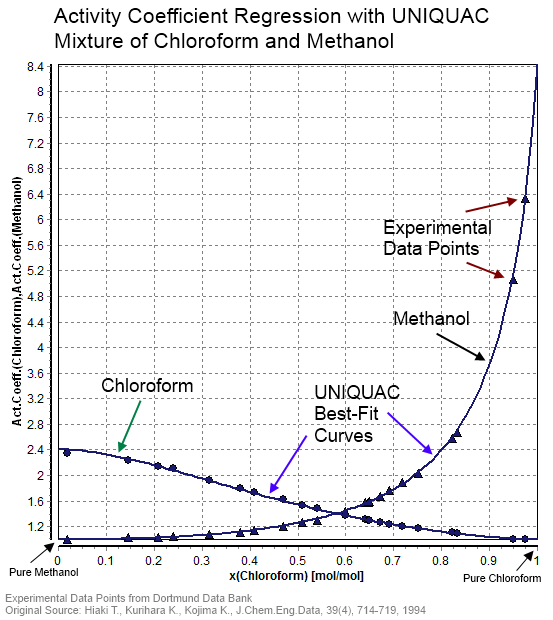 In
In
 In
In statistical thermodynamics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. Sometimes called statistical physics or statistical thermodynamics, its applicatio ...
, UNIQUAC (a portmanteau
In linguistics, a blend—also known as a blend word, lexical blend, or portmanteau—is a word formed by combining the meanings, and parts of the sounds, of two or more words together.
of universal quasichemical) is an activity coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same ( ...
model used in description of phase equilibria.
The model is a so-called lattice model and has been derived from a first order approximation of interacting molecule surfaces. The model is, however, not fully thermodynamically consistent due to its two- liquid mixture approach. In this approach the local concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'', ...
around one central molecule is assumed to be independent from the local composition around another type of molecule.
The UNIQUAC model can be considered a second generation activity coefficient because its expression for the excess Gibbs energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of work, other than pressure–volume work, that may be performed by a ther ...
consists of an entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
term in addition to an enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
term. Earlier activity coefficient models such as the Wilson equation and the non-random two-liquid model
The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model introduced by Renon
and John Prausnitz, Prausnitz in 1968 that correlates the activity coefficients \gamma_i of a compound with its mole fractions x_i in th ...
(NRTL model) only consist of enthalpy terms.
Today the UNIQUAC model is frequently applied in the description of phase equilibria (i.e. liquid–solid, liquid–liquid or liquid–vapor equilibrium). The UNIQUAC model also serves as the basis of the development of the group contribution method UNIFAC, where molecules are subdivided into functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s. In fact, UNIQUAC is equal to UNIFAC for mixtures of molecules, which are not subdivided; e.g. the binary systems water-methanol, methanol-acryonitrile and formaldehyde-DMF.
A more thermodynamically consistent form of UNIQUAC is given by the more recent COSMOSPACE and the equivalent GEQUAC model.
Equations
Like most local composition models, UNIQUAC splits excessGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
into a combinatorial and a residual contribution:
:
The calculated activity coefficients of the ''i''th component then split likewise:
:
The first is an entropic term quantifying the deviation from ideal solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
as a result of differences in molecule shape. The latter is an enthalpicHere it is assumed that the enthalpy change upon mixing can be assumed to be equal to the energy upon mixing, since the liquid excess molar volume is small and Δ ''H''ex=Δ''U''ex+''V''ex Δ''P'' ≈ Δ''U'' correction caused by the change in interacting forces between different molecules upon mixing.
Combinatorial contribution
The combinatorial contribution accounts for shape differences between molecules and affects the entropy of the mixture and is based on the lattice theory. The Stavermann–Guggenheim equation is used to approximate this term from pure chemical parameters, using the relative Van der Waals volumes ''r''''i'' and surface areas ''q''''i''It is assumed that all molecules have the same coordination number as the methylene group of an alkane, which is the reference to calculate the relative volume and surface area. of the pure chemicals: : Differentiating yields the excess entropy ''γC'', : with the volume fraction per mixture mole fraction, Vi, for the ith component given by: : The surface area fraction per mixture molar fraction, Fi, for the ith component is given by: : The first three terms on the right hand side of the combinatorial term form the Flory–Huggins contribution, while the remaining term, the Guggenhem–Staverman correction, reduce this because connecting segments cannot be placed in all direction in space. This spatial correction shifts the result of the Flory–Huggins term about 5% towards an ideal solution. The coordination number, ''z'', i.e. the number of close interacting molecules around a central molecule, is frequently set to 10. It is based on the coordination number of an methylene group in a long chain, which has in the approximation of a hexagonal close packing structure of spheres 10 intermolecular and 2 bonds.By setting qI and ri to the value an infinite long chain, infinite times the value of the methylene group, one finds with Eqn. B3 of the original paper the limiting value z=10. In the case of infinite dilution for a binary mixture, the equations for the combinatorial contribution reduce to: : This pair of equations show that molecules of same shape, i.e. same ''r'' and ''q'' parameters, have .Residual contribution
The residual, enthalpic term contains an empirical parameter, , which is determined from the binary interaction energy parameters. The expression for the residual activity coefficient for molecule i is: : with : /molis the binary interaction energy parameter. Theory defines , and , where is the interaction energy between molecules and . The interaction energy parameters are usually determined from activity coefficients, vapor-liquid, liquid-liquid, or liquid-solid equilibrium data. Usually , because the energies of evaporation (i.e. ), are in many cases different, while the energy of interaction between molecule i and j is symmetric, and therefore . If the interactions between the j molecules and i molecules is the same as between molecules i and j, there is no excess energy of mixing, . And thus . Alternatively, in some process simulation software can be expressed as follows : : . The ''C'', ''D'', and ''E'' coefficients are primarily used in fitting liquid–liquid equilibria data (with ''D'' and ''E'' rarely used at that). The ''C'' coefficient is useful for vapor-liquid equilibria data as well. The use of such an expression ignores the fact that on a molecular level the energy, , is temperature independent. It is a correction to repair the simplifications, which were applied in the derivation of the model.Applications (phase equilibrium calculations)
Activity coefficients can be used to predict simple phase equilibria (vapour–liquid, liquid–liquid, solid–liquid), or to estimate other physical properties (e.g. viscosity of mixtures). Models such as UNIQUAC allow chemical engineers to predict the phase behavior of multicomponent chemical mixtures. They are commonly used inprocess simulation
Process simulation is used for the design, development, analysis, and optimization of technical process of simulation of processes such as: chemical plants, chemical processes, environmental systems, power stations, complex manufacturing operati ...
programs to calculate the mass balance in and around separation units.
Parameters determination
UNIQUAC requires two basic underlying parameters: relative surface and volume fractions are chemical constants, which must be known for all chemicals (''q''''i'' and ''r''''i'' parameters, respectively). Empirical parameters between components that describes the intermolecular behaviour. These parameters must be known for all binary pairs in the mixture. In a quaternary mixture there are six such parameters (1–2,1–3,1–4,2–3,2–4,3–4) and the number rapidly increases with additional chemical components. The empirical parameters are obtained by a correlation process from experimental equilibrium compositions or activity coefficients, or from phase diagrams, from which the activity coefficients themselves can be calculated. An alternative is to obtain activity coefficients with a method such as UNIFAC, and the UNIFAC parameters can then be simplified by fitting to obtain the UNIQUAC parameters. This method allows for the more rapid calculation of activity coefficients, rather than direct usage of the more complex method. Remark that the determination of parameters from LLE data can be difficult depending on the complexity of the studied system. For this reason it is necessary to confirm the consistency of the obtained parameters in the whole range of compositions (including binary subsystems, experimental and calculated lie-lines, Hessian matrix, etc.).Newer developments
UNIQUAC has been extended by several research groups. Some selected derivatives are: UNIFAC, a method which permits the volume, surface and in particular, the binary interaction parameters to be estimated. This eliminates the use of experimental data to calculate the UNIQUAC parameters, extensions for the estimation of activity coefficients for electrolytic mixtures, extensions for better describing the temperature dependence of activity coefficients,Wisniewska-Goclowska B., Malanowski S.K., “A new modification of the UNIQUAC equation including temperature dependent parameters”, Fluid Phase Equilib., 180, 103–113, 2001 and solutions for specific molecular arrangements.Andreas Klamt, Gerard J. P. Krooshof, Ross Taylor “COSMOSPACE: Alternative to conventional activity-coefficient models”, AIChE J., 48(10), 2332–2349, 2004 The DISQUAC model advances UNIFAC by replacing UNIFAC's semi-empirical group-contribution model with an extension of the consistent theory of Guggenheim's UNIQUAC. By adding a "dispersive" or "random-mixing physical" term, it better predicts mixtures of molecules with both polar and non-polar groups. However, separate calculation of the dispersive and quasi-chemical terms means the contact surfaces are not uniquely defined. The GEQUAC model advances DISQUAC slightly, by breaking polar groups into individual poles and merging the dispersive and quasi-chemical terms.See also
*Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable chan ...
*Chemical thermodynamics
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measure ...
*Fugacity
In thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of chemical equilibrium. It is equal to the pressure of an ideal gas which has the same tempe ...
*MOSCED
MOSCED (short for “modified separation of cohesive energy density" model) is a thermodynamic model for the estimation of limiting activity coefficients (also known as activity coefficient at infinite dilution). From a historical point of view MOS ...
, a model for estimating limiting activity coefficients at infinite dilution
* NRTL, an alternative to UNIQUAC of the same local composition type
Notes
References
{{DEFAULTSORT:Uniquac Thermodynamic models