 |
Non-random Two-liquid Model
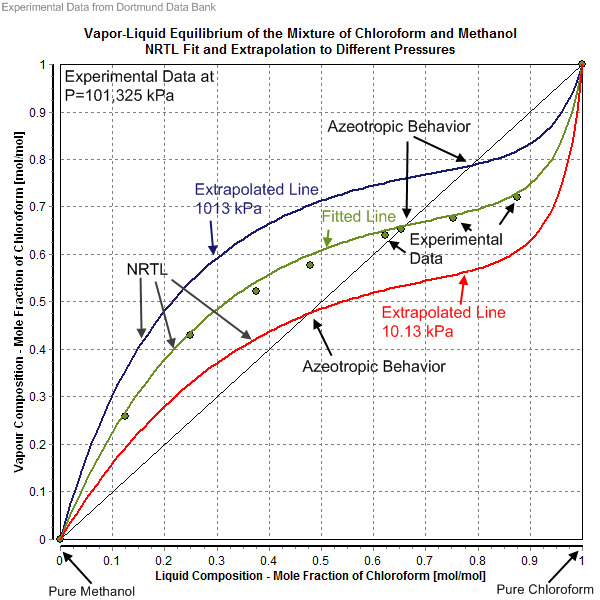
The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model introduced by Renon and John Prausnitz, Prausnitz in 1968 that correlates the activity coefficients \gamma_i of a compound with its mole fractions x_i in the liquid phase concerned. It is frequently applied in the field of chemical engineering to calculate phase equilibria. The concept of NRTL is based on the hypothesis of Wilson, who stated that the local concentration around a molecule in most mixtures is different from the bulk concentration. This difference is due to a difference between the interaction energy of the central molecule with the molecules of its own kind U_ and that with the molecules of the other kind U_. The energy difference also introduces a non-randomness at the local molecular level. The NRTL model belongs to the so-called local-composition models. Other models of this type are the Wilson model, the UNIQUAC model, and the group contribution model UNIFAC. These local-com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Antoine Equation
The Antoine equation is a class of semi-empirical correlations describing the relation between vapor pressure and temperature for pure substances. The Antoine equation is derived from the Clausius–Clapeyron relation. The equation was presented in 1888 by the French engineer (1825–1897). Equation The Antoine equation is \log_ p = A - \frac, where is the vapor pressure, is temperature (in °C or in K according to the value of ), and , and are component-specific constants. The simplified form with set to zero, \log_ p = A - \frac, is the August equation, after the German physicist Ernst Ferdinand August (1795–1870). The August equation describes a linear relation between the logarithm of the pressure and the reciprocal temperature. This assumes a temperature-independent heat of vaporization. The Antoine equation allows an improved, but still inexact description of the change of the heat of vaporization with the temperature. The Antoine equation can also be tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Thermodynamic Models
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a concise d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Physical Chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibria. Physical chemistry, in contrast to chemical physics, is predominantly (but not always) a supra-molecular science, as the majority of the principles on which it was founded relate to the bulk rather than the molecular or atomic structure alone (for example, chemical equilibrium and colloids). Some of the relationships that physical chemistry strives to understand include the effects of: # Intermolecular forces that act upon the physical properties of materials ( plasticity, tensile strength, surface tension in liquids). # Reaction kinetics on the rate of a reaction. # The identity of ions and the electrical conductivity of materials. # Surface science and electrochemistry of cell m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Simplified Molecular-input Line-entry System
Simplification, Simplify, or Simplified may refer to: Mathematics Simplification is the process of replacing a expression (mathematics), mathematical expression by an equivalent one that is simpler (usually shorter), according to a well-founded ordering. Examples include: * Computer algebra#Simplification, Simplification of algebraic expressions, in computer algebra * Simplification of boolean expressions i.e. logic optimization * Simplification by conjunction elimination in inference in logic yields a simpler, but generally non-equivalent formula * Fraction#Simplification, Simplification of fractions Science * Approximations simplify a more detailed or difficult to use process or model Linguistics * Simplification of Chinese characters * Simplified English (other) * Text simplification Music * ''Simplify'', a 1999 album by Ryan Shupe & the RubberBand * Simplified (band), a 2002 rock band from Charlotte, North Carolina * Simplified (album), ''Simplified'' (album), a 2005 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Equation Of State
In physics and chemistry, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most modern equations of state are formulated in the Helmholtz free energy. Equations of state are useful in describing the properties of pure substances and mixtures in liquids, gases, and solid states as well as the state of matter in the interior of stars. Though there are many equations of state, none accurately predicts properties of substances under all conditions. The quest for a universal equation of state has spanned three centuries. Overview At present, there is no single equation of state that accurately predicts the properties of all substances under all conditions. An example of an equation of state correlates densities of gases and liquids to temperatures and pressures, known as the ideal gas law, which is roughly accurate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Fugacity
In thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of chemical equilibrium. It is equal to the pressure of an ideal gas which has the same temperature and molar Gibbs free energy as the real gas. Fugacities are determined experimentally or estimated from various models such as a Van der Waals gas that are closer to reality than an ideal gas. The real gas pressure and fugacity are related through the dimensionless fugacity coefficient \varphi = \frac. For an ideal gas, fugacity and pressure are equal, and so . Taken at the same temperature and pressure, the difference between the molar Gibbs free energies of a real gas and the corresponding ideal gas is equal to . The fugacity is closely related to the thermodynamic activity. For a gas, the activity is simply the fugacity divided by a reference pressure to give a dimensionless quantity. This reference pressure is called the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
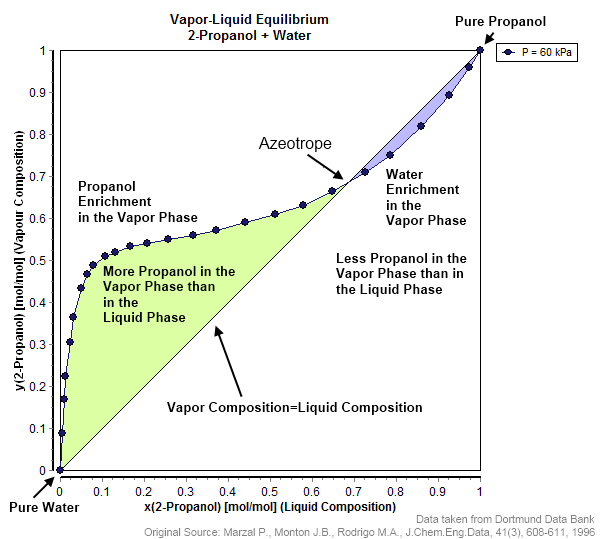 |
Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Knowing an azeotrope's behavior is important for distillation. Each azeotrope has a characteristic boiling point. The boiling point of an azeotrope is either less than the boiling point temperatures of any of its constituents (a positive azeotrope), or greater than the boiling point of any of its constituents (a negative azeotrope). For both positive and negative azeotropes, it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. For technical applications, the pressure-temperature-composition behavior of a mixture is the most important, but other important ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Dortmund Data Bank
The Dortmund Data Bank (short DDB) is a factual data bank for thermodynamic and thermophysical data. Its main usage is the data supply for process simulation where experimental data are the basis for the design, analysis, synthesis, and optimization of chemical processes. The DDB is used for fitting parameters for thermodynamic models like NRTL or UNIQUAC and for many different equations describing pure component properties, e.g., the Antoine equation for vapor pressures. The DDB is also used for the development and revision of predictive methods like UNIFAC and PSRK. Contents Mixture properties * Phase equilibria data ( vapor–liquid, liquid–liquid, solid–liquid), data on azeotropy and zeotropy * Mixing enthalpies * Gas solubilities * Activity coefficients at infinite dilution * Heat capacities and excess heat capacities * Volumes, densities, and excess volumes (volume effect of mixing) * Salt solubilities * Octanol-water partition coefficients * Criti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Miscibility Gap
A miscibility gap is a region in a phase diagram for a mixture In chemistry, a mixture is a material made up of two or more different chemical substances which can be separated by physical method. It is an impure substance made up of 2 or more elements or compounds mechanically mixed together in any proporti ... of components where the mixture exists as two or more phases – any region of composition of mixtures where the constituents are not completely miscible. The IUPAC Gold Book defines ''miscibility gap'' as "Area within the coexistence curve of an isobaric phase diagram (temperature vs composition) or an isothermal phase diagram (pressure vs composition)." A miscibility gap between isostructural phases may be described as the '' solvus'', a term also used to describe the boundary on a phase diagram between a miscibility gap and other phases. Thermodynamically, miscibility gaps indicate a maximum (''e.g.'' of Gibbs energy) in the composition range. The miscibility ga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Rowan University
Rowan University is a public research university in Glassboro, New Jersey, with a medical campus in Stratford and medical and academic campuses in Camden. Founded in 1923 as Glassboro Normal School on a site donated by 107 residents, the school was formerly known as Glassboro State College from 1958 until 1992 and Rowan College of New Jersey from 1992 to 1997. The university includes 14 colleges and schools with a total enrollment (undergraduate, graduate, and professional studies) of just over 19,600 students. Rowan offers bachelor's, master's, doctoral, and professional degree programs. It is classified among "R2: Doctoral Universities – High research activity". History In the early part of the 20th century, there was a shortage of adequately trained teachers in the state of New Jersey. It was decided to build a two-year Normal school in the southern part of the state to counter the trend. Among the candidate towns, Glassboro became the location due in no small part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
John Prausnitz
John Michael Prausnitz (born January 7, 1928) is an emeritus professor of chemical engineering at the University of California, Berkeley. Prausnitz is a member of the National Academy of Sciences and the National Academy of Engineering for contributions to the thermodynamics of phase equilibria and its application to industrial process design. In 2003, he received the National Medal of Science for his work in molecular thermodynamics. He developed many of the activity coefficient models used for the design of major chemical plants. Education Prausnitz was born in Berlin, Germany, on January 7, 1928. His father and his mother's stepfather were both Jewish doctors. In 1933, when the Nazi Party rescinded the licenses of Jewish doctors, they were able to continue to work because they had been Frontkämpfer, "front fighters," during World War I. In 1937, at age 9, Prausnitz emigrated with his parents and sister to the United States, where he had an uncle in Lynbrook, New York on Lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |