|
Bismuth(III) Sulfide
Bismuth(III) sulfide () is a chemical compound of bismuth and sulfur. It occurs in nature as the mineral bismuthinite. Synthesis Bismuth(III) sulfide can be prepared by reacting a bismuth(III) salt with hydrogen sulfide: : Bismuth (III) sulfide can also be prepared by the reaction of elemental bismuth and elemental sulfur in an evacuated silica tube at 500 °C for 96 hours. : Properties Bismuth(III) sulfide is isostructural with stibnite (stibnite is one of the forms of antimony(III) sulfide). Bismuth atoms are in two different environments, both of which have 7 coordinate Bismuth atoms, 4 in a near planar rectangle and three more distant making an irregular 7-coordination group. It can react with acids to produce the odoriferous hydrogen sulfide Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions ( anions), which results in a compound with no net electric charge (electrically neutral). The constituent ions are held together by electrostatic forces termed ionic bonds. The component ions in a salt can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic, such as ammonium () and carbonate () ions in ammonium carbonate. Salts containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases, such as sodium hydroxide and potassium oxide. Individual ions within a salt usually have multiple near neighbours, so they are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Salts usually form crystalline structures ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
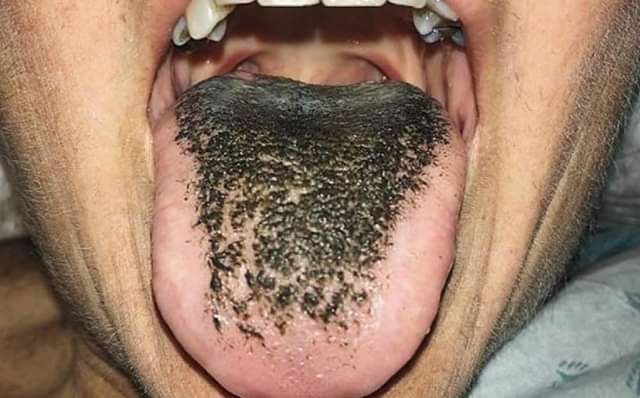
Black Hairy Tongue
Black hairy tongue syndrome (BHT) is a condition of the tongue in which the small bumps on the tongue elongate with black or brown discoloration, giving a black and hairy appearance. The appearance may be alarming, but it is a harmless condition. Predisposing factors include smoking, xerostomia (dry mouth), soft diet, poor oral hygiene and certain medications. Management is facilitated by improving oral hygiene, especially scraping or brushing the tongue. Signs and symptoms Hairy tongue largely occurs in the central part of the dorsal tongue, just anterior (in front) of the circumvallate papillae, although sometimes the entire dorsal surface may be involved. Discoloration usually accompanies hairy tongue, and may be yellow, brown or black. Apart from the appearance, the condition is typically asymptomatic, but sometimes people may experience a gagging sensation or a bad taste. There may also be associated oral malodor (intra-oral halitosis). The term "melanoglossia' is als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth Subsalicylate
Bismuth subsalicylate, sold generically as pink bismuth and under brand names including Pepto-Bismol, Pepti-Calm, and BisBacter, is a medication used to treat temporary discomfort of the stomach and gastrointestinal tract. This includes an upset stomach, heartburn, or other similar symptoms. Bismuth subsalicylate has the empirical chemical formula C7H5BiO4, and is a colloidal substance obtained by hydrolysis of bismuth salicylate (Bi(C6H4(OH)CO2)3). Medical uses As a derivative of salicylic acid, bismuth subsalicylate displays anti-inflammatory and bactericidal action. It also acts as an antacid. Mechanism of action Bismuth subsalicylate is used as an antacid and antidiarrheal, and to treat some other gastrointestinal symptoms, such as nausea. The means by which this occurs is still not well documented. It is thought to be some combination of the following: [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony(III) Sulfide
Antimony trisulfide () is found in nature as the crystalline mineral stibnite and the amorphous red mineral (actually a mineraloid) metastibnite. It is manufactured for use in safety matches, military ammunition, explosives and fireworks. It is also used as friction materials in break lining. It is very important critical primer material for military applications and tracer bullets. It also is used in the production of ruby-colored glass and in plastics as a flame retardant. Historically the stibnite form was used as a grey pigment in paintings produced in the 16th century. In 1817, the dye and fabric chemist, John Mercer discovered the non-stoichiometric compound Antimony Orange (approximate formula ), the first good orange pigment available for cotton fabric printing. Antimony trisulfide was also used as the image sensitive photoconductor in vidicon camera tubes. It is a semiconductor A semiconductor is a material with electrical conductivity between that of a conduct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stibnite
Stibnite, sometimes called antimonite, is a sulfide mineral, a mineral form of antimony trisulfide ( Sb2 S3). It is a soft, metallic grey crystalline solid with an orthorhombic space group. It is the most important source for the metalloid antimony. The name is derived from the Greek through the Latin as the former name for the mineral and the element antimony. Structure Stibnite has a structure similar to that of arsenic trisulfide, As2S3. The Sb(III) centers, which are pyramidal and three-coordinate, are linked via bent two-coordinate sulfide ions. However, some studies suggest that the actual coordination polyhedra of antimony are SbS7, with (3+4) coordination at the M1 site and (5+2) at the M2 site. Some of the secondary bonds impart cohesion and are connected with packing. Stibnite is grey when fresh, but can turn superficially black due to oxidation in air. Properties The melting point of Sb2S3 is . The band gap is 1.88 eV at room temperature and it is a pho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isostructural
Isostructural chemical compounds have similar chemical structures. " Isomorphous" when used in the relation to crystal structures is not synonymous: in addition to the same atomic connectivity that characterises isostructural compounds, isomorphous substances crystallise in the same space group and have the same unit cell dimensions. The IUCR definition used by crystallographers is: Examples include: *I- Gold(I) bromide is isostructural with gold(I) chloride *Borazine is isostructural with benzene * Indium(I) bromide is isostructural with β- thallium(I) iodide and has a distorted rock salt structure. Many minerals are isostructural when they differ only in the nature of a cation. Compounds which are isoelectronic usually have similar chemical structures. For example, methane, CH4, and the ammonium ion, NH4+, are isoelectric and are isostructural as both have a tetrahedral structure. The C-H and N-H bond lengths are different and crystal structures are completely different bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or its salts are ingested in high amounts, damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the human body produces small amounts of this sulfide and its mineral salts, and uses it as a signalling molecule. Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is commonly known as anaerobic digestio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuthinite
Bismuthinite is a mineral consisting of bismuth sulfide ( Bi2 S3). It is an important ore for bismuth. The crystals are steel-grey to off-white with a metallic luster. It is soft enough to be scratched with a fingernail and rather dense. Bismuthinite forms a series with the lead, copper, bismuth mineral aikinite ( Pb Cu Bi S3). It occurs in hydrothermal veins with tourmaline-bearing copper veins associated with granite, in some high temperature gold veins, and in recent volcanic exhalation deposits. Associated minerals include native bismuth, aikinite, arsenopyrite, stannite, galena, pyrite, chalcopyrite, tourmaline, wolframite, cassiterite and quartz. It was first reported in 1832 from the mines of Potosí, Bolivia Bolivia, officially the Plurinational State of Bolivia, is a landlocked country located in central South America. The country features diverse geography, including vast Amazonian plains, tropical lowlands, mountains, the Gran Chaco Province, w .... Refe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth(III) Oxide
Bismuth(III) oxide is a compound of bismuth, and a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite (tetragonal, much more rare), but it is usually obtained as a by-product of the smelting of copper and lead ores. Dibismuth trioxide is commonly used to produce the " Dragon's eggs" effect in fireworks, as a replacement of red lead. Structure The structures adopted by differ substantially from those of arsenic(III) oxide, , and antimony(III) oxide, .Wells, A.F. (1984) ''Structural Inorganic Chemistry''. 5th. London, England: Oxford University Press. p.890 Bismuth oxide, has five crystallographic polymorphs. The room temperature phase, α- has a monoclinic crystal structure. There are three high temperature phases, a tetragonal β-phase, a body-centred cubic γ-phase, a cubic δ- phase and an ε-phase. The room temperature α-phase has a complex structure with layers of oxygen atoms with layers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly iridescent appearance due to thin-film interference. Bismuth is both the most diamagnetic element and one of the least thermally conductive metals known. Bismuth was formerly understood to be the element with the highest atomic mass whose nuclei do not spontaneously decay. However, in 2003 it was found to be very slightly radioactive. The metal's only primordial isotope, bismuth-209, undergoes alpha decay with a half-l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |