Bismuth on:
[Wikipedia]
[Google]
[Amazon]
Bismuth is a
 Beginning with Johann Heinrich Pott in 1738,
Beginning with Johann Heinrich Pott in 1738,
 In strongly acidic
In strongly acidic

 The reported abundance of bismuth in the Earth's crust varies significantly by source from 180ppb (similar to that of silver) to 8ppb (twice as common as gold). The most important ores of bismuth are bismuthinite and bismite. Native bismuth is known from Australia, Bolivia, and China.
According to the
The reported abundance of bismuth in the Earth's crust varies significantly by source from 180ppb (similar to that of silver) to 8ppb (twice as common as gold). The most important ores of bismuth are bismuthinite and bismite. Native bismuth is known from Australia, Bolivia, and China.
According to the
 The price for pure bismuth metal was relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Before World War II, demand for bismuth was small and mainly pharmaceutical—bismuth compounds were used to treat such conditions as digestive disorders, sexually transmitted diseases and burns. Minor amounts of bismuth metal were consumed in fusible alloys for
The price for pure bismuth metal was relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Before World War II, demand for bismuth was small and mainly pharmaceutical—bismuth compounds were used to treat such conditions as digestive disorders, sexually transmitted diseases and burns. Minor amounts of bismuth metal were consumed in fusible alloys for
see "Metal Prices in the United States through 1998" for a price summary and "Historical Statistics for Mineral and Material Commodities in the United States" for production. USGS.
 Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
 * Bismuth is included in BSCCO (bismuth strontium calcium copper oxide), which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
* Bismuth telluride is a semiconductor and an excellent thermoelectric material. Bi2Te3 diodes are used in mobile refrigerators, CPU coolers, and as detectors in
* Bismuth is included in BSCCO (bismuth strontium calcium copper oxide), which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
* Bismuth telluride is a semiconductor and an excellent thermoelectric material. Bi2Te3 diodes are used in mobile refrigerators, CPU coolers, and as detectors in
Lenntech.com. Retrieved on 17 December 2011. As with lead, bismuth poisoning can result in the formation of a black deposit on the gingiva, known as a bismuth line."Bismuth line"
in ''TheFreeDictionary's Medical dictionary''. Farlex, Inc. Poisoning may be treated with dimercaprol; however, evidence for benefit is unclear. Bismuth's environmental impacts are not well known; it may be less likely to bioaccumulate than some other heavy metals, and this is an area of active research.
Bismuth
at ''
Bismuth Crystals – Instructions & Pictures
{{Authority control Chemical elements Minerals in space group 166 Native element minerals Pnictogens Post-transition metals Alchemical substances Trigonal minerals Materials that expand upon freezing Chemical elements with rhombohedral structure
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Bi and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
83. It is a post-transition metal
The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metal ...
and one of the pnictogen
, -
! colspan=2 style="text-align:left;" , ↓ Period
, -
! 2
,
, -
! 3
,
, -
! 4
,
, -
! 5
,
, -
! 6
,
, -
! 7
,
, -
, colspan="2",
----
''Legend''
A pnictogen ( or ; from "to choke" and -gen, "generator") is any ...
s, with chemical properties resembling its lighter group 15 siblings arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
and antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
. Elemental bismuth occurs naturally, and its sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
and oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
forms are important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly iridescent appearance due to thin-film interference. Bismuth is both the most diamagnetic
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagn ...
element and one of the least thermally conductive metals known.
Bismuth was formerly understood to be the element with the highest atomic mass whose nuclei do not spontaneously decay. However, in 2003 it was found to be very slightly radioactive. The metal's only primordial isotope, bismuth-209, undergoes alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
roughly a billion times longer than the estimated age of the universe.
Bismuth metal has been known since ancient times. Before modern analytical methods bismuth's metallurgical similarities to lead and tin often led it to be confused with those metals. The etymology of "bismuth" is uncertain. The name may come from mid-sixteenth-century Neo-Latin
Neo-LatinSidwell, Keith ''Classical Latin-Medieval Latin-Neo Latin'' in ; others, throughout. (also known as New Latin and Modern Latin) is the style of written Latin used in original literary, scholarly, and scientific works, first in Italy d ...
translations of the German words or , meaning 'white mass', which were rendered as or .
Bismuth compounds account for about half the global production of bismuth. They are used in cosmetics; pigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored sub ...
s; and a few pharmaceuticals, notably bismuth subsalicylate, used to treat diarrhea
Diarrhea (American English), also spelled diarrhoea or diarrhœa (British English), is the condition of having at least three loose, liquid, or watery bowel movements in a day. It often lasts for a few days and can result in dehydration d ...
. Bismuth's unusual propensity to expand as it solidifies is responsible for some of its uses, as in the casting of printing type. Bismuth, when in its elemental form, has unusually low toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacteria, bacterium, or plant, as well as the effect o ...
for a heavy metal. As the toxicity of lead and the cost of its environmental remediation
Environmental remediation is the cleanup of hazardous substances dealing with the removal, treatment and containment of pollution or contaminants from Natural environment, environmental media such as soil, groundwater, sediment. Remediation may be ...
became more apparent during the 20th century, suitable bismuth alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
s have gained popularity as replacements for lead. Presently, around a third of global bismuth production is dedicated to needs formerly met by lead.
History and etymology
Bismuth metal has been known since ancient times and it was one of the first 10 metals to have been discovered. The name ''bismuth'' dates to around 1665 and is of uncertain etymology. The name possibly comes from obsolete German ', ', ' (early 16th century), perhaps related toOld High German
Old High German (OHG; ) is the earliest stage of the German language, conventionally identified as the period from around 500/750 to 1050. Rather than representing a single supra-regional form of German, Old High German encompasses the numerous ...
' ("white"). The Neo-Latin
Neo-LatinSidwell, Keith ''Classical Latin-Medieval Latin-Neo Latin'' in ; others, throughout. (also known as New Latin and Modern Latin) is the style of written Latin used in original literary, scholarly, and scientific works, first in Italy d ...
' (coined by Georgius Agricola
Georgius Agricola (; born Georg Bauer; 24 March 1494 – 21 November 1555) was a German Humanist scholar, mineralogist and metallurgist. Born in the small town of Glauchau, in the Electorate of Saxony of the Holy Roman Empire, he was b ...
, who Latinized many German mining and technical words) is from the German ', itself perhaps from ', meaning "white mass".
The element was confused in early times with tin and lead because of its resemblance to those elements. Because bismuth has been known since ancient times, no one person is credited with its discovery. Agricola (1546) states that bismuth is a distinct metal in a family of metals including tin and lead. This was based on observation of the metals and their physical properties.
Miners in the age of alchemy also gave bismuth the name '','' or "silver being made" in the sense of silver still in the process of being formed within the Earth.
Bismuth was also known to the Incas and used (along with the usual copper and tin) in a special bronze alloy for knives.
Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish Pomerania, German-Swedish pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified the elements molybd ...
, and Torbern Olof Bergman, the distinctness of lead and bismuth became clear, and Claude François Geoffroy demonstrated in 1753 that this metal is distinct from lead and tin.
Characteristics

Physical characteristics
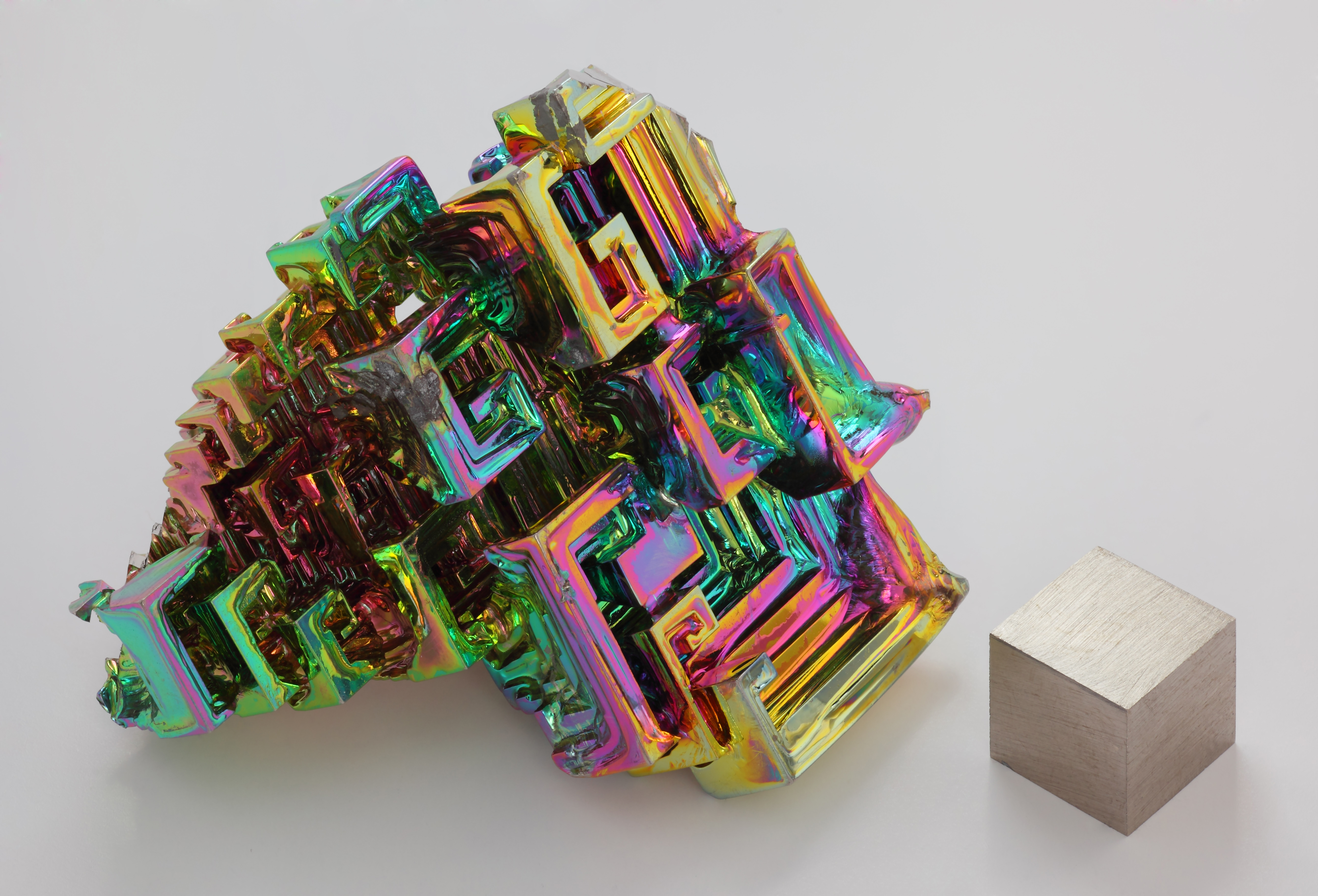
Bismuth is a brittle metal with a dark, silver-pink hue, often with an iridescentoxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
tarnish showing many colors from yellow to blue. The spiral, stair-stepped structure of bismuth crystals is the result of a higher growth rate around the outside edges than on the inside edges. The variations in the thickness of the oxide layer that forms on the surface of the crystal cause different wavelengths of light to interfere upon reflection, thus displaying a rainbow of colors. When burned in oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, bismuth burns with a blue flame and its oxide forms yellow fumes. Its toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacteria, bacterium, or plant, as well as the effect o ...
is much lower than that of its neighbors in the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
, such as lead and antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
.
No other metal is verified to be more naturally diamagnetic
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagn ...
than bismuth. ( Superdiamagnetism is a different physical phenomenon.) Of any metal, it has one of the lowest values of thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
(after manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
, neptunium and plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
) and the highest Hall coefficient
The Hall effect is the production of a potential difference (the Hall voltage) across an electrical conductor that is transverse to an electric current in the conductor and to an applied magnetic field perpendicular to the current. It was d ...
. It has a high electrical resistivity
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
. When deposited in sufficiently thin layers on a substrate, bismuth is a semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
, despite being a post-transition metal
The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metal ...
. Elemental bismuth is denser in the liquid phase than the solid, a characteristic it shares with germanium
Germanium is a chemical element; it has Symbol (chemistry), symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid or a nonmetal in the carbon group that is chemically ...
, silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
, gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875,
elemental gallium is a soft, silvery metal at standard temperature and pressure. ...
, and water. Wiberg, p. 768. Bismuth expands 3.32% on solidification; therefore, it was long a component of low-melting typesetting
Typesetting is the composition of text for publication, display, or distribution by means of arranging physical ''type'' (or ''sort'') in mechanical systems or '' glyphs'' in digital systems representing '' characters'' (letters and other ...
alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
s, where it compensated for the contraction of the other alloying components to form almost isostatic bismuth-lead eutectic alloys.
Though virtually unseen in nature, high-purity bismuth can form distinctive, colorful hopper crystals. It is relatively nontoxic and has a low melting point just above , so crystals may be grown using a household stove, although the resulting crystals will tend to be of lower quality than lab-grown crystals.
At ambient conditions, bismuth shares the same layered structure as the metallic forms of arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
and antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
, Wiberg, p. 767. crystallizing in the rhombohedral lattice. When compressed at room temperature, this Bi–I structure changes first to the monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three Vector (geometric), vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in t ...
Bi-II at 2.55 GPa, then to the tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Pri ...
Bi-III at 2.7 GPa, and finally to the body-centered cubic Bi-V at 7.7 GPa. The corresponding transitions can be monitored via changes in electrical conductivity; they are rather reproducible and abrupt and are therefore used for calibration of high-pressure equipment.
Chemical characteristics
Bismuth is stable to both dry and moist air at ordinary temperatures. When red-hot, it reacts with water to make bismuth(III) oxide. : It reacts withfluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
to form bismuth(V) fluoride at or bismuth(III) fluoride at lower temperatures (typically from Bi melts); with other halogens it yields only bismuth(III) halides. Wiberg, pp. 769–770. Greenwood, pp. 559–561. The trihalides are corrosive and easily react with moisture, forming oxyhalides with the formula BiOX.Suzuki
is a Japanese multinational mobility manufacturer headquartered in Hamamatsu, Shizuoka Prefecture, Shizuoka. It manufactures automobiles, motorcycles, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs and a va ...
, p. 9.
: (X = F, Cl, Br, I)
:
Bismuth dissolves in concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
to make bismuth(III) sulfate and sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
.Suzuki
is a Japanese multinational mobility manufacturer headquartered in Hamamatsu, Shizuoka Prefecture, Shizuoka. It manufactures automobiles, motorcycles, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs and a va ...
, p. 8.
:
It reacts with nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
to make bismuth(III) nitrate (which decomposes into nitrogen dioxide when heated).
:
It also dissolves in hydrochloric acid, but only with oxygen present.
:
Isotopes
The only primordialisotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
of bismuth, bismuth-209, was regarded as the heaviest stable nuclide, but it had long been suspected to be unstable on theoretical grounds. This was finally demonstrated in 2003, when researchers at the '' Institut d'astrophysique spatiale'' in Orsay
Orsay () is a Communes of France, commune in the Essonne Departments of France, department in Île-de-France in northern France. It is located in the southwestern suburbs of Paris, France, from the Kilometre Zero, centre of Paris.
A fortifie ...
, France, measured the alpha (α) decay half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of Bi to be (3 Bq/Mg), over times longer than the estimated age of the universe. Due to its hugely long half-life, for all known medical and industrial applications, bismuth can be treated as stable. The radioactivity is of academic interest because bismuth is one of a few elements whose radioactivity was suspected and theoretically predicted before being detected in the laboratory. Bismuth has the longest known α-decay half-life, though tellurium-128 has a double beta decay half-life of over .
Six isotopes of bismuth with short half-lives (210–215 inclusive) occur in the natural radioactive decay chains of actinium
Actinium is a chemical element; it has chemical symbol, symbol Ac and atomic number 89. It was discovered by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substa ...
, radium, thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
, and neptunium; and more have been synthesized. (Though all primordial Np has long since decayed, it is continually regenerated by (n,2n) knockout reactions on natural U.)
Commercially, bismuth-213 can be produced by bombarding radium with bremsstrahlung photons from a linear particle accelerator
A linear particle accelerator (often shortened to linac) is a type of particle accelerator that accelerates charged subatomic particles or ions to a high speed by subjecting them to a series of Oscillation, oscillating electric potentials along ...
. In 1997, an antibody conjugate with bismuth-213, which has a 45-minute half-life and α-decays, was used to treat leukemia patients. This isotope has also been tried in cancer treatment, for example, in the targeted alpha therapy (TAT) program.
Chemical compounds
Chemically, bismuth resemblesarsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
and antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
, but is much less toxic. In almost all known compounds, bismuth has oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
+3; a few have states +5 or −3.
The trioxide Greenwood, p. 553. and trisulfide can both be made from the elements, although the trioxide is extremely corrosive at high temperatures. Krüger, p. 185 The pentoxide is not stable at room temperature, and will evolve gas if heated. Both oxides form complex anions, and NaBiO3 is a strong oxidising agent. Greenwood, p. 578. The trisulfide is common in bismuth ore.
Similarly, bismuth forms all possible trihalides, but the only pentahalide is BiF5. All are Lewis acids. Bismuth forms several formally-BiI halides; these are complex salts with unusually structured polyatomic cations and anions.
 In strongly acidic
In strongly acidic aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in wat ...
solution, the Bi ion solvates to form . As pH increases, the cations polymerize until the octahedral bismuthyl complex , often abbreviated BiO+. Although bismuth oxychloride and bismuth oxynitrate have stoichiometries suggesting the ion, they are double salts instead. Bismuth nitrate (not ''oxy''nitrate) is one of the few aqueous-insoluble nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
salts.
Bismuth forms very few stable bismuthides, intermetallic compounds in which it attains oxidation state −3. The hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
spontaneously decomposes at room temperature and stabilizes only below . Sodium bismuthide has interest as a topological Dirac insulator.
Occurrence and production

 The reported abundance of bismuth in the Earth's crust varies significantly by source from 180ppb (similar to that of silver) to 8ppb (twice as common as gold). The most important ores of bismuth are bismuthinite and bismite. Native bismuth is known from Australia, Bolivia, and China.
According to the
The reported abundance of bismuth in the Earth's crust varies significantly by source from 180ppb (similar to that of silver) to 8ppb (twice as common as gold). The most important ores of bismuth are bismuthinite and bismite. Native bismuth is known from Australia, Bolivia, and China.
According to the United States Geological Survey
The United States Geological Survey (USGS), founded as the Geological Survey, is an agency of the U.S. Department of the Interior whose work spans the disciplines of biology, geography, geology, and hydrology. The agency was founded on Mar ...
(USGS), 10,200 tonnes of bismuth were produced worldwide by mining and 17,100 tonnes by refining in 2016. Since then, USGS does not provide mining data for bismuth, considering them unreliable. Globally, bismuth is mostly produced by refining, as a byproduct of extraction of other metals such as lead, copper, tin, molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
and tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
, though the refining-to-mining ratio depends on the country.
Bismuth travels in crude lead bullion (which can contain up to 10% bismuth) through several stages of refining, until it is removed by the Kroll-Betterton process which separates the impurities as slag, or the electrolytic Betts process. Bismuth will behave similarly with another of its major metals, copper. The raw bismuth metal from both processes contains still considerable amounts of other metals, foremost lead. By reacting the molten mixture with chlorine gas the metals are converted to their chlorides while bismuth remains unchanged. Impurities can also be removed by various other methods for example with fluxes and treatments yielding high-purity bismuth metal (over 99% Bi).
Price
 The price for pure bismuth metal was relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Before World War II, demand for bismuth was small and mainly pharmaceutical—bismuth compounds were used to treat such conditions as digestive disorders, sexually transmitted diseases and burns. Minor amounts of bismuth metal were consumed in fusible alloys for
The price for pure bismuth metal was relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Before World War II, demand for bismuth was small and mainly pharmaceutical—bismuth compounds were used to treat such conditions as digestive disorders, sexually transmitted diseases and burns. Minor amounts of bismuth metal were consumed in fusible alloys for fire sprinkler
A fire sprinkler or sprinkler head is the component of a fire sprinkler system that discharges water when the effects of a fire have been detected, such as when a predetermined temperature has been exceeded. Fire sprinklers are extensively used ...
systems and fuse wire. During World War II bismuth was considered a strategic material, used for solders, fusible alloys, medications and atomic research. To stabilize the market, the producers set the price at $1.25 per pound ($2.75 /kg) during the war and at $2.25 per pound ($4.96 /kg) from 1950 until 1964.
In the early 1970s, the price rose rapidly due to increasing demand for bismuth as a metallurgical additive to aluminium, iron and steel. This was followed by a decline owing to increased world production, stabilized consumption, and the recessions of 1980 and 1981–1982. In 1984, the price began to climb as consumption increased worldwide, especially in the United States and Japan. In the early 1990s, research began on the evaluation of bismuth as a nontoxic replacement for lead in ceramic glazes, fishing sinkers, food-processing equipment, free-machining brass
Brass is an alloy of copper and zinc, in proportions which can be varied to achieve different colours and mechanical, electrical, acoustic and chemical properties, but copper typically has the larger proportion, generally copper and zinc. I ...
es for plumbing applications, lubricating greases, and shot for waterfowl hunting.Suzuki
is a Japanese multinational mobility manufacturer headquartered in Hamamatsu, Shizuoka Prefecture, Shizuoka. It manufactures automobiles, motorcycles, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs and a va ...
, p. 14. Growth in these areas remained slow during the middle 1990s, in spite of the backing of lead replacement by the United States federal government, but intensified around 2005. This resulted in a rapid and continuing increase in price.Bismuth Statistics and Informationsee "Metal Prices in the United States through 1998" for a price summary and "Historical Statistics for Mineral and Material Commodities in the United States" for production. USGS.
Recycling
Most bismuth is produced as a byproduct of other metal-extraction processes including the smelting of lead, and also of tungsten and copper. Itssustainability
Sustainability is a social goal for people to co-exist on Earth over a long period of time. Definitions of this term are disputed and have varied with literature, context, and time. Sustainability usually has three dimensions (or pillars): env ...
is dependent on increased recycling, which is problematic.
It was once believed that bismuth could be practically recycled from the soldered joints in electronic equipment. Recent efficiencies in solder application in electronics mean there is substantially less solder deposited, and thus less to recycle. While recovering the silver from silver-bearing solder may remain economic, recovering bismuth is substantially less so.
Dispersed bismuth is used in certain stomach medicines ( bismuth subsalicylate), paints ( bismuth vanadate), pearlescent cosmetics ( bismuth oxychloride), and bismuth-containing bullets. Recycling bismuth from these uses is impractical.
Applications
 Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
Medicines
Bismuth is an ingredient in some pharmaceuticals, although the use of some of these substances is declining. * Bismuth subsalicylate is used to treatdiarrhea
Diarrhea (American English), also spelled diarrhoea or diarrhœa (British English), is the condition of having at least three loose, liquid, or watery bowel movements in a day. It often lasts for a few days and can result in dehydration d ...
; it is the active ingredient in such "pink bismuth" preparations as Pepto-Bismol, as well as the 2004 reformulation of Kaopectate. It is also used to treat some other gastro-intestinal diseases like shigellosis and cadmium poisoning. The mechanism of action of this substance is still not well documented, although an oligodynamic effect (toxic effect of small doses of heavy metal ions on microbes) may be involved in at least some cases. Salicylic acid from hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of the compound is antimicrobial for toxogenic ''E. coli,'' an important pathogen in traveler's diarrhea.
* A combination of bismuth subsalicylate and bismuth subcitrate is used to treat the bacteria causing peptic ulcer
Peptic ulcer disease is when the inner part of the stomach's gastric mucosa (lining of the stomach), the first part of the small intestine, or sometimes the lower esophagus, gets damaged. An ulcer in the stomach is called a gastric ulcer, while ...
s.
* Bibrocathol is an organic bismuth-containing compound used to treat eye infections.
* Bismuth subgallate, the active ingredient in Devrom, is used as an internal deodorant to treat malodor from flatulence
Flatulence is the expulsion of gas from the Gastrointestinal tract, intestines via the anus, commonly referred to as farting. "Flatus" is the medical word for gas generated in the stomach or bowels. A proportion of intestinal gas may be swal ...
and feces
Feces (also known as faeces American and British English spelling differences#ae and oe, or fæces; : faex) are the solid or semi-solid remains of food that was not digested in the small intestine, and has been broken down by bacteria in the ...
.
* Bismuth compounds (including sodium bismuth tartrate) were formerly used to treat syphilis. Arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
combined with either bismuth or mercury was a mainstay of syphilis treatment from the 1920s until the advent of penicillin in 1943.
* "Milk of bismuth" (an aqueous suspension of bismuth hydroxide and bismuth subcarbonate) was marketed as an alimentary cure-all in the early 20th century, and has been used to treat gastrointestinal disorders.
* Bismuth subnitrate (Bi5O(OH)9(NO3)4) and bismuth subcarbonate (Bi2O2(CO3)) are also used in medicine.
Cosmetics and pigments
Bismuth oxychloride (BiOCl) is sometimes used in cosmetics, as a pigment in paint for eye shadows, hair sprays and nail polishes. This compound is found as the mineral bismoclite and in crystal form contains layers of atoms (see figure above) that refract light chromatically, resulting in an iridescent appearance similar to nacre of pearl. It was used as a cosmetic inancient Egypt
Ancient Egypt () was a cradle of civilization concentrated along the lower reaches of the Nile River in Northeast Africa. It emerged from prehistoric Egypt around 3150BC (according to conventional Egyptian chronology), when Upper and Lower E ...
and in many places since. ''Bismuth white'' (also "Spanish white") can refer to either bismuth oxychloride or bismuth oxynitrate (BiONO3), when used as a white pigment. Bismuth vanadate is used as a light-stable non-reactive paint pigment (particularly for artists' paints), often as a replacement for the more toxic cadmium sulfide yellow and orange-yellow pigments. The most common variety in artists' paints is a lemon yellow, visually indistinguishable from its cadmium-containing alternative.
Electronics
Transistors
Bismuth-basedtransistors
A transistor is a semiconductor device used to Electronic amplifier, amplify or electronic switch, switch electrical signals and electric power, power. It is one of the basic building blocks of modern electronics. It is composed of semicondu ...
has been claimed to enable smaller, faster, and more energy-efficient transistors than traditional silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
. Bismuth offers a small bandgap and high electron mobility. It has topological insulator states, conducting along its surface/edges while still insulating internally. It can produce two-dimensional(2D) semiconductor materials, enabling thinner and higher-performance devices. Such 2D bismuth materials support sub-nanometer channel lengths, surpassing silicon's practical limits. However, bismuth's anisotropic heat transport can complicate chip design.
Bismuth telluride () has been investigated for use in thermoelectric transistors that use temperature gradients (e.g., via laser illumination) to generate electricity, yielding 0.7093 μW in experimental setups. They operate by leveraging the Seebeck effect, using a temperature difference to drive charge carrier movement.
Bismuth oxyselenide ( and ) have been investigated for use in field-effect transistors (FETs). These 2D materials exhibit high electron mobility
In solid-state physics, the electron mobility characterizes how quickly an electron can move through a metal or semiconductor when pushed or pulled by an electric field. There is an analogous quantity for Electron hole, holes, called hole mobilit ...
(e.g., 10–15 cm2 V⁻¹ s⁻¹) and stability in air. One study reported that these materials enabled transistors that were 40% faster and 10% more efficient than Intel’s 3 nm chips.
Bismuth can reduce contact resistance when paired with 2D semiconductors such as . This eliminates the Schottky barrier—a common efficiency issue in metal-semiconductor interfaces.
Metal and alloys
Bismuth is used in alloys with other metals such as tin and lead. Wood's metal, an alloy of bismuth, lead, tin, and cadmium, is used in automatic sprinkler systems for fires. It forms the largest part (50%) of Rose's metal, afusible alloy
A fusible alloy is a metal alloy capable of being easily fused, i.e. easily meltable, at relatively low temperatures. Fusible alloys are commonly, but not necessarily, eutectic alloys.
Sometimes the term "fusible alloy" is used to describe alloy ...
, which also contains 25–28% lead and 22–25% tin. It was also used to make bismuth bronze, which was used during the Bronze Age
The Bronze Age () was a historical period characterised principally by the use of bronze tools and the development of complex urban societies, as well as the adoption of writing in some areas. The Bronze Age is the middle principal period of ...
, having been found in Inca knives at Machu Picchu.
Lead replacement
The density difference between lead (11.32 g/cm3) and bismuth (9.78 g/cm3) is small enough that for manyballistics
Ballistics is the field of mechanics concerned with the launching, flight behaviour and impact effects of projectiles, especially weapon munitions such as bullets, unguided bombs, rockets and the like; the science or art of designing and acceler ...
and weighting applications, bismuth can substitute for lead. For example, it can replace lead as a dense material in fishing sinkers. It has been used as a replacement for lead in shot, bullets and less-lethal riot gun ammunition. The Netherlands, Denmark, England, Wales, the United States, and many other countries now prohibit the use of lead shot for the hunting of wetland birds, as many birds are prone to lead poisoning
Lead poisoning, also known as plumbism and saturnism, is a type of metal poisoning caused by lead in the body. Symptoms may include abdominal pain, constipation, headaches, irritability, memory problems, infertility, numbness and paresthesia, t ...
owing to mistaken ingestion of lead (instead of small stones and grit) to aid digestion, or even prohibit the use of lead for all hunting, such as in the Netherlands. Bismuth-tin alloy shot is one alternative that provides similar ballistic performance to lead.
Bismuth, as a dense element of high atomic weight, is used in bismuth-impregnated latex shields to shield from X-ray in medical examinations, such as CTs, mostly as it is considered non-toxic.
The European Union's Restriction of Hazardous Substances Directive (RoHS) for reduction of lead has broadened bismuth's use in electronics as a component of low-melting point solders, as a replacement for traditional tin-lead solders. Its low toxicity will be especially important for solders to be used in food processing equipment and copper water pipes, although it can also be used in other applications including those in the automobile industry, in the European Union, for example.
Bismuth has been evaluated as a replacement for lead in free-machining brass
Brass is an alloy of copper and zinc, in proportions which can be varied to achieve different colours and mechanical, electrical, acoustic and chemical properties, but copper typically has the larger proportion, generally copper and zinc. I ...
es for plumbing applications, although it does not equal the performance of leaded steels.
Other metal uses and specialty alloys
Many bismuthalloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
s have low melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
s and are found in specialty applications such as solder
Solder (; North American English, NA: ) is a fusible alloy, fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces aft ...
s. Many automatic sprinklers, electric fuses, and safety devices in fire detection and suppression systems contain the eutectic In19.1-Cd5.3-Pb22.6-Sn8.3-Bi44.7 alloy that melts at This is a convenient temperature since it is unlikely to be exceeded in normal living conditions. Low-melting alloys, such as Bi-Cd-Pb-Sn alloy which melts at , are also used in automotive and aviation industries. Before deforming a thin-walled metal part, it is filled with a melt or covered with a thin layer of the alloy to reduce the chance of breaking. Then the alloy is removed by submerging the part in boiling water. Krüger, p. 183.
Bismuth is used to make free-machining steels and free-machining aluminium alloys for precision machining properties. It has similar effect to lead and improves the chip breaking during machining. The shrinking on solidification in lead and the expansion of bismuth compensate each other and therefore lead and bismuth are often used in similar quantities. Similarly, alloys containing comparable parts of bismuth and lead exhibit a very small change (on the order 0.01%) upon melting, solidification or aging. Such alloys are used in high-precision casting, e.g. in dentistry, to create models and molds. Bismuth is also used as an alloying agent in production of malleable irons and as a thermocouple material.
Bismuth is also used in aluminium-silicon cast alloys to refine silicon morphology. However, it indicated a poisoning effect on modification of strontium
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to ...
. Some bismuth alloys, such as Bi35-Pb37-Sn25, are combined with non-sticking materials such as mica, glass and enamels because they easily wet them allowing to make joints to other parts. Addition of bismuth to caesium enhances the quantum yield of caesium cathodes. Krüger, p. 184. Sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plas ...
of bismuth and manganese powders at produces a permanent magnet and magnetostrictive material, which is used in ultrasonic generators and receivers working in the 10–100 kHz range and in magnetic and holographic memory devices.Suzuki
is a Japanese multinational mobility manufacturer headquartered in Hamamatsu, Shizuoka Prefecture, Shizuoka. It manufactures automobiles, motorcycles, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs and a va ...
, p. 15.
Other uses as compounds
 * Bismuth is included in BSCCO (bismuth strontium calcium copper oxide), which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
* Bismuth telluride is a semiconductor and an excellent thermoelectric material. Bi2Te3 diodes are used in mobile refrigerators, CPU coolers, and as detectors in
* Bismuth is included in BSCCO (bismuth strontium calcium copper oxide), which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
* Bismuth telluride is a semiconductor and an excellent thermoelectric material. Bi2Te3 diodes are used in mobile refrigerators, CPU coolers, and as detectors in infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
spectrophotometers.
* Bismuth oxide, in its delta form, is a solid electrolyte for oxygen. This form normally breaks down below a high-temperature threshold, but can be electrodeposited well below this temperature in a highly alkaline solution.
* Bismuth germanate is a scintillator, widely used in X-ray and gamma ray detectors.
* Bismuth vanadate is an opaque yellow pigment used by some artists' oil, acrylic, and watercolor paint companies, primarily as a replacement for the more toxic cadmium sulfide yellows in the greenish-yellow (lemon) to orange-toned yellow range. It performs practically identically to the cadmium pigments, such as in terms of resistance to degradation from UV exposure, opacity, tinting strength, and lack of reactivity when mixed with other pigments. The most commonly used variety by artists' paint makers is lemon in color. In addition to being a replacement for several cadmium yellows, it also serves as a non-toxic visual replacement for the older chromate pigments made with zinc, lead, and strontium. If a green pigment and barium sulfate (for increased transparency) are added it can also serve as a replacement for barium chromate, which possesses a more greenish cast than the others. In comparison with lead chromate, it does not blacken due to hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
in the air (a process accelerated by UV exposure) and possesses a particularly brighter color than them, especially the lemon, which is the most translucent, dull, and fastest to blacken due to the higher percentage of lead sulfate required to produce that shade. It is also used, on a limited basis due to its cost, as a vehicle paint pigment. Bismuth vanadate can also be used as electrocatalyst for hydrogen peroxide synthesis.
* Bismuth tungstate can be used as photocatalyst for removal of phenolic compounds as well as for hydrogen generation.
* Bismuth molybdate is a catalyst for propylene oxidation as well as photocatalyst.
* A catalyst for making acrylic fibers.
* As an electrocatalyst in the conversion of CO2 to CO.
* Ingredient in lubricating greases.
* In crackling microstars ( dragon's eggs) in pyrotechnics
Pyrotechnics is the science and craft of creating fireworks, but also includes safety matches, oxygen candles, Pyrotechnic fastener, explosive bolts (and other fasteners), parts of automotive airbags, as well as gas-pressure blasting in mining, q ...
, as the oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
, subcarbonate or subnitrate.
*As catalyst for the fluorination of arylboronic pinacol esters through a Bi(III)/Bi(V) catalytic cycle, mimicking transition metals in electrophilic fluorination.
Toxicology and ecotoxicology
:''See also bismuthia, a rare dermatological condition that results from the prolonged use of bismuth.'' Scientific literature indicates that some of the compounds of bismuth are less toxic to humans via ingestion than other heavy metals (lead, arsenic, antimony, etc.) presumably due to the comparatively low solubility of bismuth salts. Itsbiological half-life
Biological half-life (elimination half-life, pharmacological half-life) is the time taken for concentration of a drug, biological substance (such as a medication) to decrease from its maximum concentration (chemistry), concentration (Cmax (pharm ...
for whole-body retention is reported to be 5 days but it can remain in the kidney for years in people treated with bismuth compounds.
Bismuth poisoning can occur and has according to some reports been common in relatively recent times.Data on Bismuth's health and environmental effectsLenntech.com. Retrieved on 17 December 2011. As with lead, bismuth poisoning can result in the formation of a black deposit on the gingiva, known as a bismuth line."Bismuth line"
in ''TheFreeDictionary's Medical dictionary''. Farlex, Inc. Poisoning may be treated with dimercaprol; however, evidence for benefit is unclear. Bismuth's environmental impacts are not well known; it may be less likely to bioaccumulate than some other heavy metals, and this is an area of active research.
See also
* Bismuth minerals * Arsenic-bismuthNotes
References
Cited sources
. * * * *External links
Bismuth
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
Bismuth Crystals – Instructions & Pictures
{{Authority control Chemical elements Minerals in space group 166 Native element minerals Pnictogens Post-transition metals Alchemical substances Trigonal minerals Materials that expand upon freezing Chemical elements with rhombohedral structure