|
Berkelium Dioxide
Berkelium(IV) oxide, also known as berkelium dioxide, is a chemical compound with the formula BkO2. This compound slowly decays to californium(IV) oxide. It can be converted to berkelium(III) oxide by hydrogen reduction at 600 °C. :2BkO2 + H2 → Bk2O3 + H2O Production Berkelium(IV) oxide is produced by burning berkelium Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National ... metal in air at 1200 °C. It can also be produced by reacting berkelium(III) oxide with oxygen at 600 °C. References {{Oxides Berkelium compounds Oxides Fluorite crystal structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium(IV) Sulfide
Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium. The major isotope of berkelium, 249Bk, is synthesized in minute quantities in dedicated high-flux nuclear reactors, mainly at the Oak Ridge National Laboratory in Tennessee, United States, and at the Research Institute of Atomic Reactors in Dimitrovgrad, Russia. The longest-lived and second-most important isotope, 247Bk, can be synthesized via irradiation of 244Cm with high-energy alpha particles. Just over one gram of berkelium has been produced in the United States since 1967. There is no practical application of berkelium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
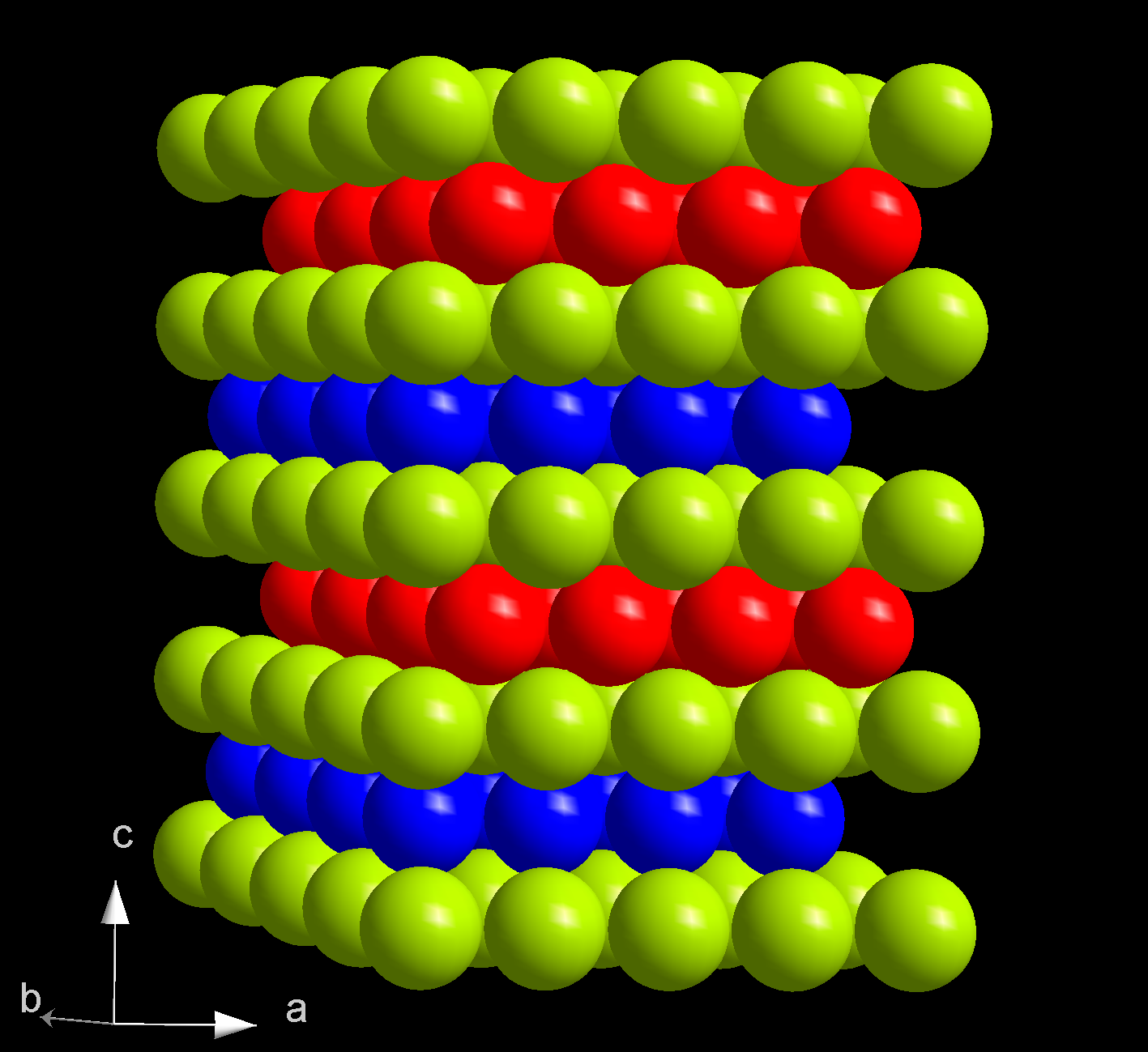
Americium(IV) Oxide
Americium dioxide (AmO2) is a black compound of americium. In the solid state, AmO2 adopts a fluorite structure (like CaF2). It is used as a source of alpha particles. Historical context The demand for americium dioxide stems from the difficulty of storing the element americium as a solution of americium(III) chloride because the alpha radiation and hydrochloric acid decomposes storage containers over time. To solve the liquid storage problem, scientists at Oak Ridge National Laboratory devised a synthesis to turn liquid americium–acid solution into a precipitated form of americium for safer handling and more efficient storage. Synthesis Synthesis of americium dioxide, as described by the Oak Ridge National Laboratory in 1960, starts by dissolving americium in hydrochloric acid, and then neutralizing the excess acid with ammonium hydroxide (). Then, saturated oxalic acid solution () is added to the now neutralized solution to precipitate dull pink americium(III) oxalate crystals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Curium(IV) Oxide
Curium(IV) oxide is an inorganic chemical compound of curium and oxygen with the chemical formula . Since all isotopes of curium are man-made, the compound does not occur in nature. Synthesis *Curium(IV) oxide can be prepared directly from the elements. Metallic curium is annealed in air or in an oxygen atmosphere: :: * Curium(III) hydroxide and curium(III) oxalate are also usually used for this purpose: :: :: *Another way is the reaction of curium(III) oxide in an oxygen atmosphere at 650 °C: :: Physical properties Curium(IV) oxide forms black crystals. Insoluble in water. The compound crystals are of the cubic crystal system In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties o ..., the fluorite structure in the space group ''Fm''3''m''. Chemical properties The compound reacts w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Californium(IV) Oxide
Californium(IV) oxide is a binary inorganic compound of californium and oxygen with the formula . __TOC__ Synthesis Californium dioxide is produced by oxidizing californium with molecular and atomic oxygen at high pressure. Physical properties Californium(IV) oxide is a black-brown solid that has a cubic fluorite crystal structure The fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure. Many compounds, notabl ... with a lattice parameter, the distance between unit cells in the crystal, of 531.0 ± 0.2 pm. References Californium compounds Oxides Fluorite crystal structure {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium(III) Oxide
Berkelium(III) oxide is a binary inorganic compound of berkelium and oxygen with the chemical formula . Synthesis Berkelium(III) oxide can be prepared from berkelium(IV) oxide by reduction with hydrogen Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...: :: Physical properties The compound forms a yellow-green solid with a melting point of 1920 °C. It forms a body-centered cubic crystal lattice with a = 1088.0 ± 0.5 pm. Insoluble in water. References Oxides Berkelium compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Californium(IV) Oxide
Californium(IV) oxide is a binary inorganic compound of californium and oxygen with the formula . __TOC__ Synthesis Californium dioxide is produced by oxidizing californium with molecular and atomic oxygen at high pressure. Physical properties Californium(IV) oxide is a black-brown solid that has a cubic fluorite crystal structure The fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure. Many compounds, notabl ... with a lattice parameter, the distance between unit cells in the crystal, of 531.0 ± 0.2 pm. References Californium compounds Oxides Fluorite crystal structure {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium(III) Oxide
Berkelium(III) oxide is a binary inorganic compound of berkelium and oxygen with the chemical formula . Synthesis Berkelium(III) oxide can be prepared from berkelium(IV) oxide by reduction with hydrogen Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...: :: Physical properties The compound forms a yellow-green solid with a melting point of 1920 °C. It forms a body-centered cubic crystal lattice with a = 1088.0 ± 0.5 pm. Insoluble in water. References Oxides Berkelium compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium
Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium. The major isotope of berkelium, 249Bk, is synthesized in minute quantities in dedicated high-flux nuclear reactors, mainly at the Oak Ridge National Laboratory in Tennessee, United States, and at the Research Institute of Atomic Reactors in Dimitrovgrad, Russia. The longest-lived and second-most important isotope, 247Bk, can be synthesized via irradiation of 244Cm with high-energy alpha particles. Just over one gram of berkelium has been produced in the United States since 1967. There is no practical application of berkeli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium Compounds
Berkelium forms a number of chemical compounds, where it normally exists in an Oxidation number, oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous solution, aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid.Peterson, p. 55Holleman, p. 1956 Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxides
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of (called a passivation layer) that protects the foil from further oxidation.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry Oxides are extraordinarily diverse in terms of stoichiometries (the measurable relationship between reactants and chemical equations of an equation or reaction) and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |