|
Ansamycins
Ansamycins is a family of bacterial secondary metabolites that show antimicrobial activity against many Gram-positive and some Gram-negative bacteria, and includes various compounds, including streptovaricins and rifamycins. In addition, these compounds demonstrate antiviral activity towards bacteriophages and poxviruses. They are somewhat similar in structure to macrolide antibiotics, but because they have a lactam instead of a lactone, they do not belong in the class of macrolides. __TOC__ Structure They are named ansamycins (from the Latin ansa, ''handle'') because of their unique structure, which consists of an aromatic moiety bridged by an aliphatic chain. The main difference between various derivatives of ansamycins is the aromatic moiety, which can be a naphthalene ring or a naphthoquinone ring as in rifamycin and the naphthomycins. Another variation consists of benzene or a benzoquinone ring system as in geldanamycin or ansamitocin. Ansamycins were first discovered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptovaricins
Streptovaricins are a group of structurally related macrolide antibiotics. They belong to the larger class of antibiotics known as ansamycins. References Macrolide antibiotics Ansamycins {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthomycin
Naphthomycins are a group of closely related antimicrobial chemical compounds isolated from ''Streptomyces''. They are considered a subclass of ansamycin Ansamycins is a family of bacterial secondary metabolites that show antimicrobial activity against many Gram-positive and some Gram-negative bacteria, and includes various compounds, including streptovaricins and rifamycins. In addition, these ...s. Members include: * Naphthomycin A * Naphthomycin B * Naphthomycin C * Naphthomycin D * Naphthomycin E * Naphthomycin F * Naphthomycin G * Naphthomycin H * Naphthomycin I * Naphthomycin J * Naphthomycin K * Naphthomycin L * Naphthomycin M * Naphthomycin N Chemical structures File:Naphthomycin A.svg, Naphthomycin A File:Naphthomycin B.svg, Naphthomycin B File:Naphthomycin C.svg, Naphthomycin C File:Naphthomycin D.svg, Naphthomycin D File:Naphthomycin E.svg, Naphthomycin E File:Naphthomycin F.svg, Naphthomycin F File:Naphthomycin G.svg, Naphthomycin G References {{reflist An ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geldanamycin
Geldanamycin is a 1,4-benzoquinone ansamycin Antitumor agent, antitumor antibiotic that inhibits the function of Hsp90 (Heat Shock Protein 90) by binding to the unusual ADP/ATP-binding pocket of the protein. HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, angiogenesis and oncogenesis. Geldanamycin induces the degradation of proteins that are mutated or overexpressed in tumor cells such as v-Src, Bcr-Abl, p53, and ERBB2. This effect is mediated via HSP90. Despite its potent antitumor potential, geldanamycin presents several major drawbacks as a drug candidate such as hepatotoxicity, further, Jilani ''et al.''. reported that geldanamycin induces the apoptosis of erythrocytes under physiological concentrations. These side effects have led to the development of geldanamycin analogues, in particular analogues containing a derivatisation at the 17 position: * 17-AAG * 17-DMAG Biosynthesis Geldanamycin was original ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinomycete
The Actinomycetales is an order of Actinomycetota. A member of the order is often called an actinomycete. Actinomycetales are generally gram-positive and anaerobic and have mycelia in a filamentous and branching growth pattern. Some actinomycetes can form rod- or coccoid-shaped forms, while others can form spores on aerial hyphae. Actinomycetales bacteria can be infected by bacteriophages, which are called actinophages. Actinomycetales can range from harmless bacteria to pathogens with resistance to antibiotics. Reproduction Actinomycetales have 2 main forms of reproduction: spore formation and hyphae fragmentation. During reproduction, Actinomycetales can form conidiophores, sporangiospores, and oidiospores. In reproducing through hyphae fragmentation, the hyphae formed by Actinomycetales can be a fifth to half the size of fungal hyphae, and bear long spore chains. Presence and associations Actinomycetales can be found mostly in soil and decaying organic matter, as well as in l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethers
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactams
A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words '' lactone'' + ''amide''. Nomenclature Greek prefixes in alphabetical order indicate ring size. This ring-size nomenclature stems from the fact that hydrolysis of an α-lactam gives an α-amino acid and that of a β-Lactam gives a β-amino acid, and so on. Synthesis General synthetic methods are used for the organic synthesis of lactams. Beckmann rearrangement Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement. Schmidt reaction Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclohexanone with hydrazoic acid, forms ε - Caprolactum, which upon treatment with excess acid forms Cardiazole, a heart stimulant. Cyclization of amino acids Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
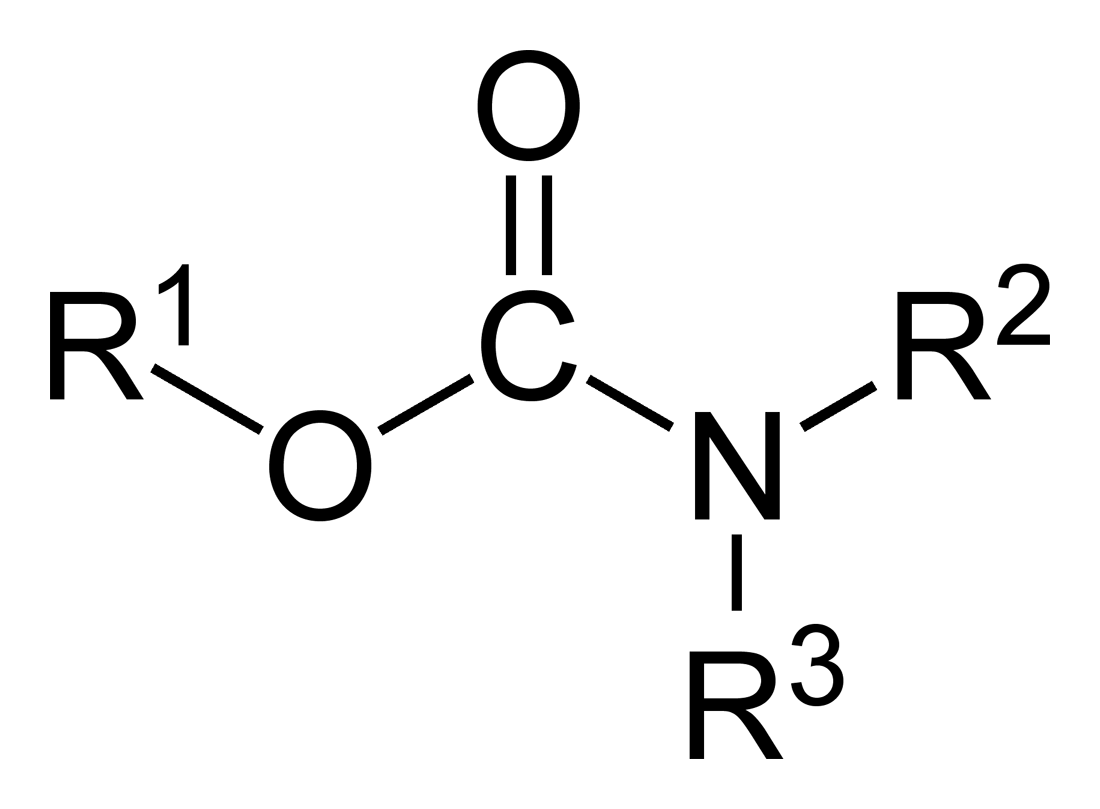
Carbamates
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose repeat units are joined by carbamate like groups are an important family of plastics, the polyurethanes. See for clarification. Properties While carbamic acids are unstable, many carbamate esters and salts are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyketides
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (, or its reduced forms) and methylene () groups: . First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity. History Naturally produced polyketides by various plants and organisms have been used by humans since before studies on them began in the 19th and 20th century. In 1893, J. Norman Collie synthesized detectable amounts of orcinol by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prokaryotes
A prokaryote (; less commonly spelled procaryote) is a single-celled organism whose cell lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Greek (), meaning 'before', and (), meaning 'nut' or 'kernel'. In the earlier two-empire system arising from the work of Édouard Chatton, prokaryotes were classified within the empire Prokaryota. However, in the three-domain system, based upon molecular phylogenetics, prokaryotes are divided into two domains: Bacteria and Archaea. A third domain, Eukaryota, consists of organisms with nuclei. Prokaryotes evolved before eukaryotes, and lack nuclei, mitochondria, and most of the other distinct organelles that characterize the eukaryotic cell. Some unicellular prokaryotes, such as cyanobacteria, form colonies held together by biofilms, and large colonies can create multilayered microbial mats. Prokaryotes are asexual, reproducing via binary fission. Horizontal gene transfer is comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |