|
3,3'-Dimethoxybenzidine
''o''-Dianisidine is an organic compound with the formula CH3O)(H2N)C6H3sub>2. A colorless or white solid, it is a bifunctional compound derived via the benzidine rearrangement from ''o''-anisidine. ''o''-Dianisidine is a precursor to some azo dyes by formation of the bis(diazonium) derivative, which is coupled to diverse aromatic compounds. Some commercial dyes derived from ''o''-dianisidine include C. I. Direct Blue 1, 15, 22, 84, and 98.. ''o''-Dianisidine is also used in assaying activity of peroxidase in lab. The general reaction of a peroxidase is as follows. :ROOR' + \overset + 2H+ -> ce + R'OH Where the ROOR' can be hydrogen peroxide, and the electron donor be ''o''-dianisidine. Safety The manufacture and degradation of ''o''-dianisidine, like other benzidene derivatives, has attracted regulatory attention. It is also used as a reagent in biochemistry in testing for peroxide In chemistry, peroxides are a group of Chemical compound, compounds with the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IARC Group 2B Carcinogens
IARC group 2B substances, mixtures and exposure circumstances are those that have been classified as "possibly carcinogenic to humans" by the International Agency for Research on Cancer (IARC) as This category is used when there is level of evidence, limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals. It may also be used when there is insufficient evidence of carcinogenicity in humans but sufficient evidence in experimental animals. In some cases, an agent, mixture, or exposure circumstance with inadequate evidence of carcinogenicity in humans but limited evidence in experimental animals, combined with supporting evidence from other relevant data, may be included in this group. This list focuses on the hazard linked to the agents. This means that the carcinogenic agents are capable of causing cancer, but this does not take their risk into account, which is the probability of causing a cancer given the level o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzidine Rearrangement
Benzidine ( trivial name), also called 1,1'- biphenyl-4,4'-diamine (systematic name), is an organic compound with the formula (C6H4NH2)2. It is an aromatic amine. It is a component of a test for cyanide. Related derivatives are used in the production of dyes. Benzidine has been linked to bladder and pancreatic cancer. Synthesis and properties Benzidine is prepared in a two step process from nitrobenzene. First, the nitrobenzene is converted to 1,2-diphenylhydrazine, usually using iron powder as the reducing agent. Treatment of this hydrazine with mineral acids induces a rearrangement reaction to 4,4'-benzidine. Smaller amounts of other isomers are also formed. The benzidine rearrangement, which proceeds intramolecularly, is a classic mechanistic puzzle in organic chemistry. : The conversion is described as a ,5 sigmatropic reaction. : In terms of its physical properties, 4,4'-benzidine is poorly soluble in cold water but can be recrystallized from hot water, where it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-Anisidine
''o''-Anisidine (2-anisidine) is an organic compound with the formula CH3OC6H4NH2. A colorless liquid, commercial samples can appear yellow owing to air oxidation. It is one of three isomers of the methoxy-containing aniline derivative. Production and use It is prepared via methanolysis of 2-chloronitrobenzene: :NaOCH3 + ClC6H4NO2 → CH3OC6H4NO2 + NaCl The resulting ''o''-nitroanisole is reduced to ''o''-anisidine. ''o''-Anisidine is used in the manufacture of dyes. It is nitrated to give 4-nitroanisidine. It is also a precursor to ''o''-dianisidine. One special use is as a heartwood indicator. An acid solution of ''o''-anisidine is diazotized by adding a sodium nitrite solution. This mixture is applied to the wood and by reaction with polyphenols in the heartwood a reddish brown azo dye is formed. : Safety and environmental aspects ''o''-Anisidine is a dangerous pollutant from the production of dyes. It is listed as RCRA hazardous waste Hazardous waste is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct dyes, substantive dyes are employed for cellulose-based textil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazonium Salt
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent, compound where R is hydrogen, is diazenylium. Structure and general properties Arene derivatives According to X-ray crystallography the linkage is linear in typical diazonium salts. The bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å, which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p''K''a values compared to their unsubstituted counterparts. The p''K''a of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group raises the ionization constant ''K'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
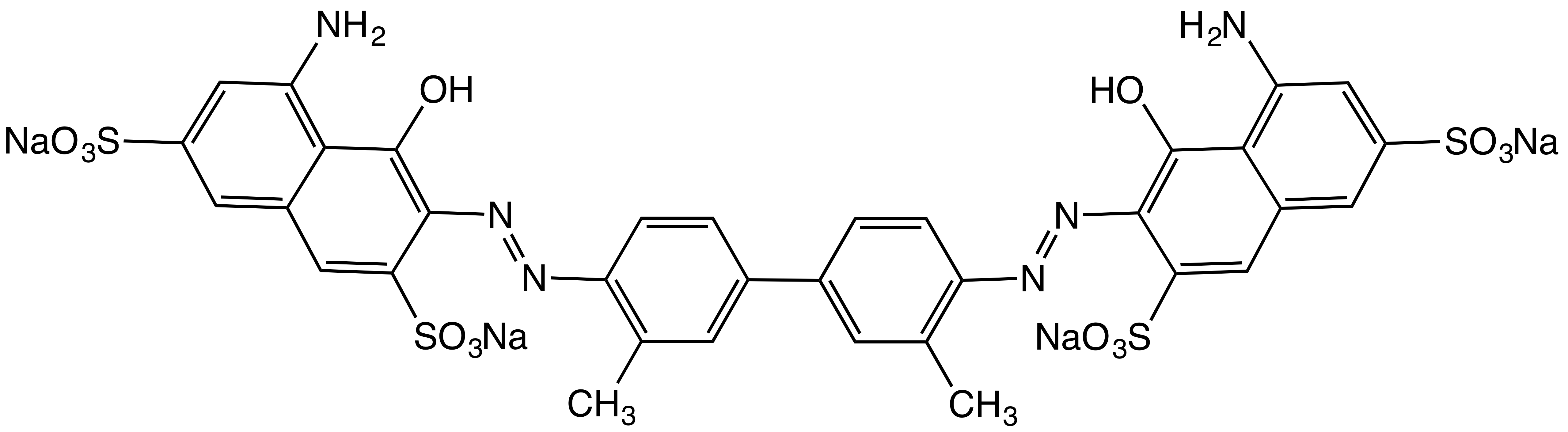
Direct Blue 1
Direct Blue 1 is an organic compound that is one of many azo dye Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C l ...s. This salt is used as a substantive dye for textiles with high contents of cellulose, i.e. cotton. It is prepared by the azo coupling of the aminonaphthalene and diazotized derivative of ''o''-dianisidine.Klaus Hunger, Peter Mischke, Wolfgang Rieper, Roderich Raue, Klaus Kunde, Aloys Engel: "Azo Dyes" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2005, Wiley-VCH, Weinheim.. References Azo dyes Naphthalenes Phenol ethers Sulfonates Organic sodium salts {{Ether-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Direct Blue 15
Direct Blue 15 is an organic compound that is classified as an azo dye. It is a dark blue water soluble solid. It is a popular substantive dye, which means that it useful for dying cotton and related cellulosic materials. It is produced by azo coupling In organic chemistry, an azo coupling is an organic reaction, reaction between a diazonium compound () and another aromatic compound that produces an azo compound (). In this electrophilic aromatic substitution reaction, the aryldiazonium cation ... of o-dianisidine with the appropriate naphthalene disulfonate.. References {{Reflist Azo dyes Naphthalenesulfonic acids 1-Naphthols Naphthylamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Direct Blue 22
Direct may refer to: Mathematics * Directed set, in order theory * Direct limit of (pre), sheaves * Direct sum of modules, a construction in abstract algebra which combines several vector spaces Computing * Direct access (other), a method of accessing data in a database * Direct connect (other), various methods of telecommunications and computer networking * Direct memory access, access to memory by hardware subsystems independently of the CPU Entertainment * Direct (Tower of Power album), ''Direct'' (Tower of Power album) * Direct (Vangelis album), ''Direct'' (Vangelis album) * Direct (EP), ''Direct'' (EP), by The 77s Other uses * Direct (music symbol), a music symbol used in music notation that is similar to a catchword in literature * Nintendo Direct, an online presentation frequently held by Nintendo * Mars Direct, a proposal for a crewed mission to Mars * DIRECT, a proposed space shuttle-derived launch vehicle * DirectX, a proprietary dynamic medi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxidase
Peroxidases or peroxide reductases ( EC numberbr>1.11.1.x are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides, and should not be confused with other enzymes that ''produce'' peroxide, which are often oxidases. Functionality Peroxidases typically catalyze a reaction of the form: :ROOR' + \overset + 2H+ -> ce + R'OH Optimal substrates For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides. Peroxidases can contain a heme cofactor in their active sites, or alternately redox-active cysteine or selenocysteine residues. The nature of the electron donor is very dependent on the structure of the enzyme. * For example, horseradish peroxidase can use a variety of organic compounds as electron donors and acceptors. Horseradish peroxidase has an accessible active site, and many compounds can re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or "high-test peroxide", decomposes explosively when heated and has been used as both a monopropellant and an oxidizer in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly into water and elemental oxygen when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a Stabilizer (chemistry), stabilizer in a weakly acidic solution in an opaque bottle. Hydrogen peroxide is found in biological systems including the human body. Enzymes that u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |