|
Peroxidase
Peroxidases or peroxide reductases ( EC numberbr>1.11.1.x are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides, and should not be confused with other enzymes that ''produce'' peroxide, which are often oxidases. Functionality Peroxidases typically catalyze a reaction of the form: :ROOR' + \overset + 2H+ -> ce + R'OH Optimal substrates For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides. Peroxidases can contain a heme cofactor in their active sites, or alternately redox-active cysteine or selenocysteine residues. The nature of the electron donor is very dependent on the structure of the enzyme. * For example, horseradish peroxidase can use a variety of organic compounds as electron donors and acceptors. Horseradish peroxidase has an accessible active site, and many compounds can re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione Peroxidase
Glutathione peroxidase (GPx) () is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water. Glutathione peroxidase was discovered in 1957 by Gordon C. Mills. Reaction The main reaction that glutathione peroxidase catalyzes is: : 2GSH + H2O2 → GS–SG + 2H2O where GSH represents reduced monomeric glutathione, and GS–SG represents glutathione disulfide. The mechanism involves oxidation of the selenol of a selenocysteine residue by hydrogen peroxide. This process gives the derivative with a selenenic acid (RSeOH) group. The selenenic acid is then converted back to the selenol by a two step process that begins with reaction with GSH to form the GS-SeR and water. A second GSH molecule reduces the GS-SeR intermediate back to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GPX1
Glutathione peroxidase 1, also known as GPx1, is an enzyme that in humans is encoded by the ''GPX1'' gene on chromosome 3. This gene encodes a member of the glutathione peroxidase family. Glutathione peroxidase functions in the detoxification of hydrogen peroxide, and is one of the most important antioxidant enzymes in humans. Structure This gene encodes a member of the glutathione peroxidase family, consisting of eight known glutathione peroxidases (GPx1-8) in humans. Mammalian Gpx1 (this gene), Gpx2, Gpx3, and Gpx4 have been shown to be selenium-containing enzymes, whereas Gpx6 is a selenoprotein in humans with cysteine-containing homologues in rodents. In selenoproteins, the 21st amino acid selenocysteine is inserted in the nascent polypeptide chain during the process of translational recoding of the UGA stop codon. In addition to the UGA-codon, a cis-acting element in the mRNA, called SECIS, binds SBP2 to recruit other proteins, such as eukaryotic elongation factor seleno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADH Peroxidase
In enzymology, a NADH peroxidase () is an enzyme that catalysis, catalyzes the chemical reaction :NADH + H+ + H2O2 \rightleftharpoons NAD+ + 2 H2O The presumed function of NADH peroxidase is to inactivate H2O2 generated within the cell, for example by glycerol-3-phosphate oxidase during glycerol metabolism or dismutation of superoxide, before the H2O2 causes damage to essential cellular components. The 3 substrate (biochemistry), substrates of this enzyme are nicotinamide adenine dinucleotide, NADH, hydrogen ion, H+, and hydrogen peroxide, H2O2, whereas its two product (chemistry), products are nicotinamide adenine dinucleotide, NAD+ and water, H2O. It employs one cofactor (biochemistry), cofactor, flavin adenine dinucleotide, FAD, however no discrete FADH2 intermediate has been observed. This enzyme belongs to the family of oxidoreductases, specifically those acting on a peroxide as acceptor (peroxidases). The List of enzymes, systematic name of this enzyme class is NADH:hydrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Peroxidase
In enzymology, a manganese peroxidase () is an enzyme that catalyzes the chemical reaction :2 Mn(II) + 2 H+ + H2O2 \rightleftharpoons 2 Mn(III) + 2 H2O The 3 substrates of this enzyme are Mn(II), H+, and H2O2, whereas its two products are Mn(III) and H2O. This enzyme belongs to the family of oxidoreductases, to be specific those acting on a peroxide as acceptor (peroxidases). The systematic name of this enzyme class is Mn(II):hydrogen-peroxide oxidoreductase. Other names in common use include peroxidase-M2, and Mn-dependent (NADH-oxidizing) peroxidase. It employs one cofactor, heme. This enzyme needs Ca2+ for activity. White rot fungi secrete this enzyme to aid lignin degradation. Discovery and characterization Manganese peroxidase (commonly referred to as MnP) was discovered in 1985 simultaneously by the research groups of Michael H. Gold and Ronald Crawford in the fungus ''Phanerochaete chrysosporium''. The protein was genetically sequenced in ''P. chrysoporium'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Animal Heme-dependent Peroxidases
Animal heme-dependent peroxidases is a family of peroxidases. Peroxidases are found in bacteria, fungi, plants and animals. On the basis of sequence similarity, a number of animal heme peroxidases can be categorized as members of a superfamily: myeloperoxidase (MPO); eosinophil peroxidase (EPO); lactoperoxidase (LPO); thyroid peroxidase (TPO); prostaglandin H synthase (PGHS); and peroxidasin. Function Myeloperoxidase (MPO) plays a major role in the oxygen-dependent microbicidal system of neutrophils. EPO from eosinophilic granulocytes participates in immunological reactions, and potentiates tumor necrosis factor (TNF) production and hydrogen peroxide release by human monocyte-derived macrophages. MPO (and possibly EPO) primarily use Cl−ions and H2O2 to form hypochlorous acid (HOCl), which can effectively kill bacteria or parasites. In secreted fluids, LPO catalyses the oxidation of thiocyanate ions (SCN−) by H2O2, producing the weak oxidizing agent hypothiocyanite (OSCN−), wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome C Peroxidase
Cytochrome ''c'' peroxidase, or CCP, is a water-soluble heme-containing enzyme of the peroxidase family that takes reducing equivalents from cytochrome ''c'' and reduces hydrogen peroxide to water: :CCP + H2O2 + 2 ferrocytochrome ''c'' + 2H+ → CCP + 2H2O + 2 ferricytochrome ''c'' CCP can be derived from aerobically grown yeast strains and can be isolated in both native and recombinant forms with high yield from ''Saccharomyces cerevisiae.'' The enzyme’s primary function is to eliminate toxic radical molecules produced by the cell which are harmful to biological systems. It works to maintain low concentration levels of hydrogen peroxide, which is generated by the organism naturally through incomplete oxygen reduction. When glucose levels in fast growing yeast strains are exhausted, the cells turn to respiration which raises the concentration of mitochondrial H2O2. In addition to its peroxidase activity, it acts as a sensor and a signaling molecule to exogenous H2O2, which acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haem Peroxidase
Haem peroxidases (or heme peroxidases) are haem-containing enzymes that use hydrogen peroxide Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ... as the electron acceptor to catalyse a number of oxidative reactions. Most haem peroxidases follow the reaction scheme: : Fe3+ + H2O2 \rightleftharpoons e4+=O' (Compound I) + H2O : e4+=O' + substrate --> e4+=O (Compound II) + oxidized substrate : e4+=O + substrate --> Fe3+ + H2O + oxidized substrate In this mechanism, the enzyme reacts with one equivalent of H2O2 to give e4+=O' (compound I). This is a two-electron oxidation/reduction reaction in which H2O2 is reduced to water, and the enzyme is oxidized. One oxidizing equivalent resides on iron, giving the oxyferryl intermediate, and in many peroxidases the porphyrin (R) is oxidi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloperoxidase
Haloperoxidases are peroxidases that are able to mediate the oxidation of halides by hydrogen peroxide. Both halides and hydrogen peroxide are widely available in the environment. Mechanistic and thermodynamic considerations Halogenations of organic compounds by free halogens () is generally favorable process. It is practiced industrially on a big scale for example. In nature, however, free halogens do not exist in appreciable amounts. The combination of hydrogen peroxide, which is widely produced by aerobic life, and halide anions provides the equivalent of . The oxidation of these anions by hydrogen peroxide is slow in the absence of enzymes. These enzymes are called haloperoxidases. The reaction that they catalyze is: : From the perspective of thermodynamics, the Nernst equation confirms that hydrogen peroxide can oxidize chloride (E°= 1.36 V), bromide (E°= 1.09 V), and iodide (E°= 0.536 V) from a thermodynamic perspective under natural conditions, i.e., a temperature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
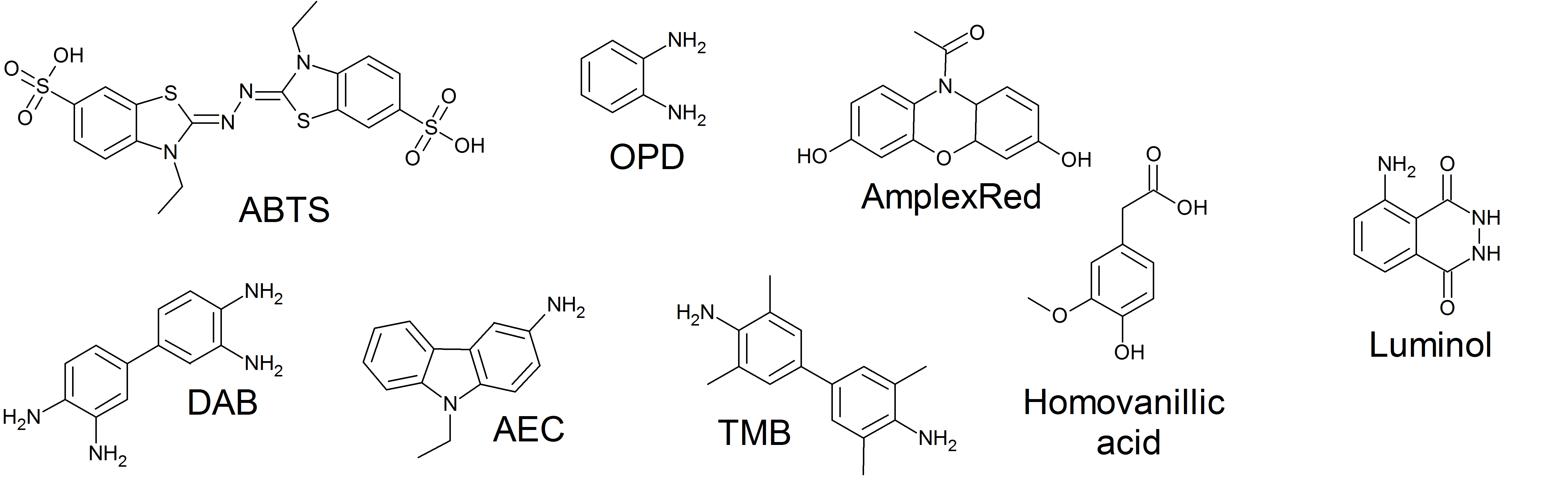
Horseradish Peroxidase
The enzyme horseradish peroxidase (HRP), found in the roots of horseradish, is used extensively in biochemistry applications. It is a metalloenzyme with many isoforms, of which the most studied type is C. It catalyzes the oxidation of various organic substrates by hydrogen peroxide. Structure The structure of the enzyme was first solved by X-ray crystallography in 1997; and has since been solved several times with various substrates. It is a large alpha-helix, alpha-helical glycoprotein which binds heme as a redox Cofactor (biochemistry), cofactor. Substrates Alone, the HRP enzyme, or conjugates thereof, is of little value; its presence must be made visible using a Substrate (biochemistry), substrate that, when Redox, oxidized by HRP using hydrogen peroxide as the oxidizing agent, yields a characteristic color change that is detectable by spectrophotometric methods. Numerous substrates for horseradish peroxidase have been described and commercialized to exploit the desir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium Bromoperoxidase
Vanadium bromoperoxidases are a kind of enzymes called haloperoxidases. Its primary function is to remove hydrogen peroxide which is produced during photosynthesis Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ... from in or around the cell. By producing hypobromous acid (HOBr) a secondary reaction with dissolved organic matter, what results is the bromination of organic compounds that are associated with the defense of the organism. These enzymes produce the bulk of natural organobromine compounds in the world. Vanadium bromoperoxidases are one of the few classes of enzymes that requires vanadium. The active site features a vanadium oxide center attached to the protein via one histidine side chain and a collection of hydrogen bonds to the oxide ligands. Occurrence and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DyP-type Peroxidase Family
In molecular biology, the DyP-type peroxidase family is a family of haem peroxidase enzymes. Haem peroxidases were originally divided into two superfamilies, namely, the animal peroxidases and the plant peroxidases (which are subdivided into class I, II and III), which include fungal (class II) and bacterial peroxidases. The DyP (for dye de-colourising peroxidase) family constitutes a novel class of haem peroxidase. Because these enzymes were derived from fungal sources, the DyP family was thought to be structurally related to the class II secretory fungal peroxidases. However, the DyP family exhibits only low sequence similarity to classical fungal peroxidases, such as LiP and MnP, and does not contain the conserved proximal and distal histidines and an essential arginine found in other plant peroxidase superfamily members. DyP proteins have several characteristics that distinguish them from all other peroxidases, including a particularly wide substrate specificity, a lack of homo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prosthetic group, component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 Vinyl group, vinyl and 2 propionic acid side chains. Heme is biosynthesized in both the bone marrow and the liver. Heme plays a critical role in multiple different redox reactions in mammals, due to its ability to carry the oxygen molecule. Reactions include oxidative metabolism (cytochrome c oxidase, succinate dehydrogenase), xenobiotic detoxification via cytochrome P450 pathways (including Drug metabolism, metabolism of some drugs), gas sensing (Guanylate cyclase, guanyl cyclases, nitric oxide synthase), and microRNA processing (DGCR8). Heme is a coordination complex "consisting of an iron ion coordinated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |