|
O-Anisidine
''o''-Anisidine (2-anisidine) is an organic compound with the formula CH3OC6H4NH2. A colorless liquid, commercial samples can appear yellow owing to air oxidation. It is one of three isomers of the methoxy-containing aniline derivative. Production and use It is prepared via methanolysis of 2-chloronitrobenzene: :NaOCH3 + ClC6H4NO2 → CH3OC6H4NO2 + NaCl The resulting ''o''-nitroanisole is reduced to ''o''-anisidine. ''o''-Anisidine is used in the manufacture of dyes. It is nitrated to give 4-nitroanisidine. It is also a precursor to ''o''-dianisidine. One special use is as a heartwood indicator. An acid solution of ''o''-anisidine is diazotized by adding a sodium nitrite solution. This mixture is applied to the wood and by reaction with polyphenols in the heartwood a reddish brown azo dye is formed. : Safety and environmental aspects ''o''-Anisidine is a dangerous pollutant from the production of dyes. It is listed as RCRA hazardous waste Hazardous waste is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IARC Group 2A Carcinogens
IARC group 2A agents are substances and exposure circumstances that have been classified as probable carcinogens by the International Agency for Research on Cancer (IARC). This designation is applied when there is limited evidence of carcinogenicity in humans, as well as ''sufficient evidence'' of carcinogenicity in experimental animals. In some cases, an agent may be classified in this group when there is ''inadequate evidence'' of carcinogenicity in humans along with ''sufficient evidence'' of carcinogenicity in experimental animals and ''strong evidence'' that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this group solely on the basis of ''limited evidence'' of carcinogenicity in humans. This list is focusing on the hazard linked to the agents. This means that the carcinogenic agents are capable of causing cancer, but this does not take their risk into account, which is the probability of causing a can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heartwood
Wood is a structural tissue/material found as xylem in the stems and roots of trees and other woody plants. It is an organic materiala natural composite of cellulosic fibers that are strong in tension and embedded in a matrix of lignin that resists compression. Wood is sometimes defined as only the secondary xylem in the stems of trees, or more broadly to include the same type of tissue elsewhere, such as in the roots of trees or shrubs. In a living tree, it performs a mechanical-support function, enabling woody plants to grow large or to stand up by themselves. It also conveys water and nutrients among the leaves, other growing tissues, and the roots. Wood may also refer to other plant materials with comparable properties, and to material engineered from wood, woodchips, or fibers. Wood has been used for thousands of years for fuel, as a construction material, for making tools and weapons, furniture and paper. More recently it emerged as a feedstock for the production of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hazardous Waste
Hazardous waste is waste that must be handled properly to avoid damaging human health or the environment. Waste can be hazardous because it is Toxicity, toxic, Chemical reaction, reacts violently with other chemicals, or is Corrosion, corrosive, among other traits. As of 2022, humanity produces 300-500 million metric tons of hazardous waste annually. Some common examples are electronics, batteries, and paints. An important aspect of managing hazardous waste is safe disposal. Hazardous waste can be stored in hazardous waste landfills, burned, or recycled into something new. Managing hazardous waste is important to achieve worldwide sustainability. Hazardous waste is regulated on national scale by national governments as well as on an international scale by the United Nations (UN) and international treaties. Types Universal wastes Universal wastes are a special category of hazardous wastes that (in the U.S.) generally pose a lower threat relative to other hazardous wastes, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
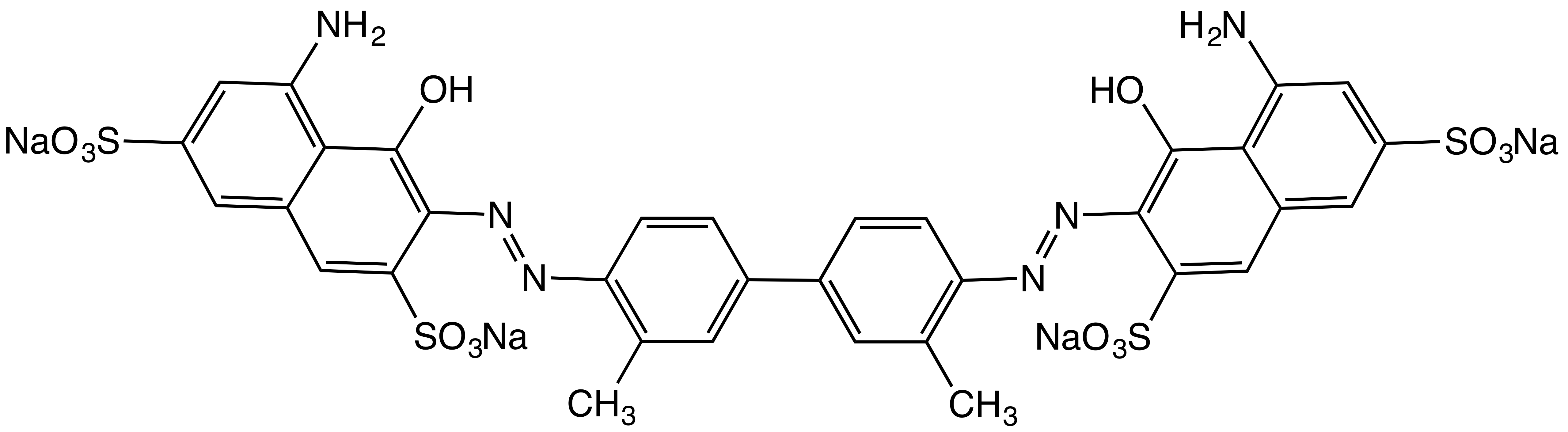
Direct Blue 15
Direct Blue 15 is an organic compound that is classified as an azo dye. It is a dark blue water soluble solid. It is a popular substantive dye, which means that it useful for dying cotton and related cellulosic materials. It is produced by azo coupling In organic chemistry, an azo coupling is an organic reaction, reaction between a diazonium compound () and another aromatic compound that produces an azo compound (). In this electrophilic aromatic substitution reaction, the aryldiazonium cation ... of o-dianisidine with the appropriate naphthalene disulfonate.. References {{Reflist Azo dyes Naphthalenesulfonic acids 1-Naphthols Naphthylamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct dyes, substantive dyes are employed for cellulose-based textil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyphenols
Polyphenols () are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include phenolic acids, flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments. Etymology The name derives from the Ancient Greek word (, meaning "many, much") and the word ‘phenol’ which refers to a chemical structure formed by attachment of an aromatic benzenoid (phenyl) ring to a hydroxyl (-OH) group (hence the ''-ol'' suffix). The term "polyphenol" has been in use at least since 1894. Definition Polyphenols are natural products with "one or several hydroxyl groups on aromatic rings", including four principal classes: phenolic acids, flavonoids, stilbenes, and lignans. Flavonoids can be grouped as flavones, flavonols, flavanols, flavanones, isoflavones, proanthocyanidins, and anthocyanins. Particularly abundant flavanoids in foods are catechin (tea, fruits), hespereti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Coupling
In organic chemistry, an azo coupling is an reaction between a diazonium compound () and another aromatic compound that produces an azo compound (). In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activated carbon (usually from an arene, which is called coupling agent), serves as a nucleophile. Classical coupling agents are phenols and naphthols. Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate. Uses of the reaction Aromatic azo compounds tend to be brightly colored due to their extended conjugated systems. Many are useful dyes (see azo dye). Important azo dyes include methyl red and pigment red 170. Azo printing exploits this reaction as well. In this case, the diazonium ion is degraded by light, leaving a latent image in undegraded diazonium salt which is made to react with a phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-Nitroanisole
''o''-Nitroanisole is an organic compound with the formula CH3OC6H4NO2. Three isomers of nitroanisole exist, but the o-isomer is the most commercially important. It is a colorless liquid. It is prepared by treatment of ''o''-chloronitrobenzene with sodium methoxide Sodium methoxide is the simplest sodium alkoxide. With the formula , it is a white solid, which is formed by the deprotonation of methanol. It is a widely used reagent in industry and the laboratory. It is also a dangerously caustic base. ...: :NaOCH3 + ClC6H4NO2 → CH3OC6H4NO2 + NaCl The resulting 2-chloronitrobenzene can be reduced to o-anisidine, which is a precursor to dyes. References {{DEFAULTSORT:Nitroanisole, o- Nitrobenzene derivatives IARC Group 2A carcinogens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |