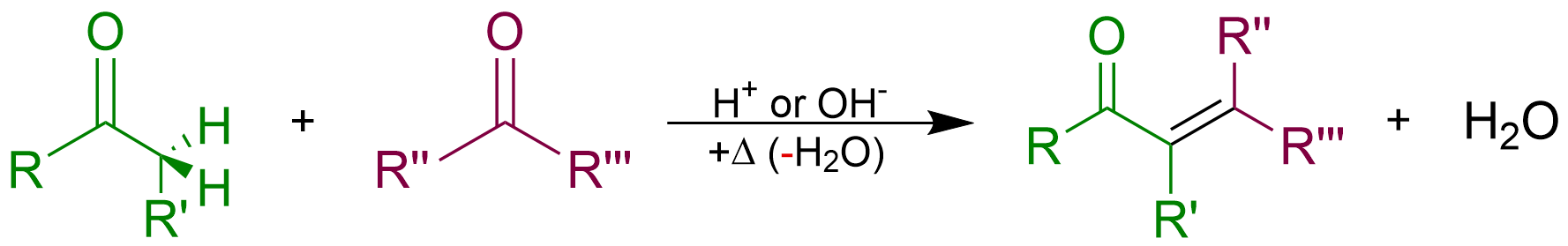|
2-Cumaranone
2-Coumaranone (sometimes also called 2-Cumaranone) is a bicyclic heteroaromatic compound in which a six-membered benzene ring is Annulation, annulated with a five-membered γ-butyrolactone ring. The 2(3''H'')-benzofuranone can also be considered as a lactone of (2-hydroxyphenyl)acetic acid. The benzofuranone basic structure is the basis of some natural products – such as rosmadial, which is isolatable from Rosemary Oil, rosemary oil, and some substances with high pharmacological activity, such as griseofulvin and rifampicin. Furthermore, 2-cumaranone is utilized as a starting material for the preparation of chemiluminescent and fluorescent dyes, for synthetic pharmaceutical agents, like the antiarrhythmic drug dronedarone, and especially for the fungicide azoxystrobin. Occurrence and synthesis In 1884, Adolf von Baeyer and Paul Fritsch (chemist), Paul Fritsch disclosed the synthesis of 2-coumaranone, which they described as the lactone of ''o''-oxyphenylacetic acid, through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicyclic
A bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring atoms are carbons), or heterocyclic (the rings' atoms consist of at least two elements), like DABCO. Moreover, the two rings can both be aliphatic (''e.g.'' decalin and norbornane), or can be aromatic (''e.g.'' naphthalene), or a combination of aliphatic and aromatic (''e.g.'' tetralin). Three modes of ring junction are possible for a bicyclic compound: * In spiro compounds, the two rings share only one single atom, the spiro atom, which is usually a quaternary carbon. An example of a spirocyclic compound is the photochromic switch spiropyran. * In fused/condensed bicyclic compounds, two rings share two adjacent atoms. In other words, the rings share one covalent bond, ''i.e.'' the bridgehead atoms are directly connected (''e.g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Fritsch (chemist)
Paul Fritsch (25 February 1901 – 22 September 1970) was a French featherweight professional boxing, boxer who competed in the early 1920s. In 1920 he became the first French boxer to win an Olympic title, defeating teammate Jean Gachet in the final, despite losing to Gachet at the national championships before the Olympics. After more than 300 amateur bouts, Fritsch turned professional in 1921. He fought approximately 100 more bouts, but never won a major title. He retired from boxing in 1929 due to a retinal detachment and became a car salesman. 1920 Olympic results Below is the record of Paul Fritsch, a French featherweight boxer who competed at the 1920 Antwerp Olympics: * Round of 32: bye * Round of 16: defeated George Etcell (USA) * Quarterfinal: defeated Paul Erdal (Norway) * Semifinal: defeated Edoardo Garzena (Italy) * Final: defeated Jean Gachet (France) Note: In 1920 a country could have more than one boxer per weight classification References 1901 birth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction equation is as follows (where the Rs can be H) Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or aldol (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, the first step is formally an addition reaction rather than a condensation reaction bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
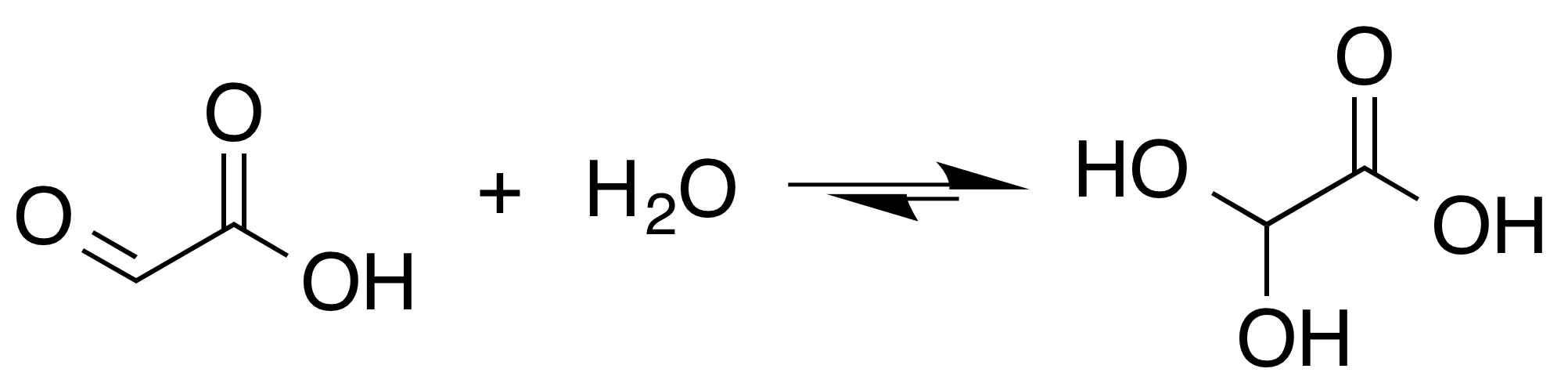
Glyoxylic Acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature The structure of glyoxylic acid is shown as having an aldehyde functional group. The aldehyde is only a minor component of the form most prevalent in some situations. Instead, glyoxylic acid often exists as a hydrate or a cyclic dimer (chemistry), dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: Dihydroxyacetic acid has been characterized by X-ray crystallography. : In aqueous solution, this monohydrate exists in equilibrium with a hemiacylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of cyclohexanone assume a pale yellow color. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Millions of tonnes are produced annually, mainly as a precursor to nylon. History and synthesis The compound was discovered by in 1888 among the products of AC electrolysis of slightly acidified water solutions of phenol. He named it hydrophenoketone and correctly suggested that phenol was first hydrogenated by electrolytic hydrogen to cyclohexanol, which he wasn't able to isolate, and then oxidized by electrolytic oxygen. Laboratory synthesis Cyclohexanone can be prepared from cyclohexanol by oxidation with chromium trioxide ( Jones oxidation). An alternative method utilizes the safer and more readily avai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Rearrangement
The Claisen rearrangement is a powerful carbon–carbon chemical bond, bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl Vinyl group, vinyl ether will initiate a Sigmatropic reaction, [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation with Δ(Δf''H'') ca. . Mechanism The Claisen rearrangement is an exothermic, concerted (bond cleavage and recombination) pericyclic reaction. Woodward–Hoffmann rules show a suprafacial, stereospecific reaction pathway. The kinetics are of the first order and the whole transformation proceeds through a highly ordered cyclic transition state and is intramolecular. Crossover experiment (chemistry), Crossover experiments eliminate the possibility of the rearrangement occurring via an intermolecular reaction mechanism and are consistent with an intramolecular process. There are substantial solvent effects observed in the Claisen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-bromopropene
Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, perfumes and other organic compounds. Allyl bromide is a colorless liquid, although commercial samples appear yellow or brown. It is an irritant and a potentially dangerous alkylating agent. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use. Preparation Hydrohalogenation Allyl bromide is produced commercially from allyl alcohol and hydrobromic acid: :CH2=CHCH2OH + HBr → CH2=CHCH2Br + H2O It can also be prepared by the halogen-exchange reaction between allyl chloride and hydrobromic acid or by the allylic bromination of propene. Reactions and uses Allyl bromide is an electrophilic alkylating agent. It reacts with nucleophiles, such as amines, carbanions, alkoxides, etc., to introduce the allyl group: :CH2=CHCH2Br + Nu− → CH2=CHCH2Nu + Br− (Nu− is a nucleophile) It is used in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceuti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the Saturated and unsaturated compounds, unsaturated bonds are Bond cleavage, cleaved with ozone (). Multiple carbon–carbon bond are replaced by carbonyl () groups, such as aldehydes, ketones, and carboxylic acids. The reaction is predominantly applied to alkenes, but alkynes and azo compounds are also susceptible to cleavage. The outcome of the reaction depends on the type of multiple bond being oxidized and the Work-up (chemistry), work-up conditions. Detailed procedures have been reported. Ozonolysis of alkenes Alkenes can be oxidized with ozone to form Alcohol (chemistry), alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at until the solution takes on a characteristic blue color, which is due to unreacted ozone. Industry however recommends temperatures near . This color change indicates complete consumption of the alk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Group
A methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ... atom, which is connected to the remainder of the molecule by two single bonds. The group may be represented as or , where the '>' denotes the two bonds. This stands in contrast to a situation where the carbon atom is bound to the rest of the molecule by a double bond, which is preferably called a methylidene group, represented . Formerly the methylene name was used for both isomers. The name “ methylene bridge“ can be used for the single-bonded isomer, to emphatically exclude methylidene. The distinction is often important, because the double bond is chemically different from two single bonds. The methylene group should be d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |