|
2-Aminobenzothiazole
2-Aminobenzothiazole is the organic compound with the formula . It is related to the parent benzothiazole, but with an amino group at the unique methyne position on the thiazole ring. As confirmed by X-ray crystallography, it is a planar molecule, which exists as the amine tautomer. 2-Aminobenzothiazoles are often prepared by cyclization of 2-bromo-substituted arylthioureas. : Isothiocyanic acid, which can be generated in situ from sodium thiocyanate, adds to anilines to also afford 2-aminobenzothiazoles. Many other methods are available. Diazotization of 2-aminobenzothiazoles gives diazonium salts. These salts undergo azo coupling with aniline Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...s. In this way some are prepared some useful dyes such as Basic Blue 54. Referen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzothiazole
Benzothiazole, or more specifically 1,3-benzothiazole, is an aromatic heterocyclic compound with the chemical formula . It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole. It has a sulfurous odor and meaty flavor. The three structural isomers of benzothizaole are 1,3-benzothiazole, 1,2-benzothiazole and 2,1-benzothiazole. Structure and reactivity Benzothiazoles consist of a 5-membered 1,3- thiazole ring fused to a benzene ring. The nine atoms of the bicycle and the attached substituents are coplanar. The heterocyclic core of the molecule is readily substituted at the methyne (CH) centre in the thiazole ring. Thiazole is electron-withdrawing. Synthesis and biosynthesis Benzothiazoles are typically prepared by treatment of 2-mercaptoaniline. For example, acid chlorides are effective: :C6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Thiocyanate
Sodium thiocyanate (sometimes called sodium sulphocyanide) is the chemical compound with the formula NaSCN. This colorless deliquescent salt is one of the main sources of the thiocyanate anion. As such, it is used as a precursor for the synthesis of pharmaceuticals and other specialty chemicals. Thiocyanate salts are typically prepared by the reaction of cyanide with elemental sulfur: :8 NaCN + S8 → 8 NaSCN Sodium thiocyanate crystallizes in an orthorhombic cell. Each Na+ center is surrounded by three sulfur and three nitrogen ligands provided by the triatomic thiocyanate anion. It is commonly used in the laboratory as a test for the presence of Fe3+ ions. Applications in chemical synthesis Sodium thiocyanate is employed to convert alkyl halides into the corresponding alkylthiocyanates. Treatment of isopropyl bromide with sodium thiocyanate in a hot ethanolic solution affords isopropyl thiocyanate. Protonation of sodium thiocyanate affords isothiocyanic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzothiazoles
Benzothiazole, or more specifically 1,3-benzothiazole, is an aromatic heterocyclic compound with the chemical formula . It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole. It has a sulfurous odor and meaty flavor. The three structural isomer, structural isomers of benzothizaole are 1,3-benzothiazole, 1,2-benzothiazole and 2,1-benzothiazole. Structure and reactivity Benzothiazoles consist of a 5-membered 1,3-thiazole ring Annulation, fused to a benzene ring. The nine atoms of the bicycle and the attached substituents are coplanar. The heterocyclic core of the molecule is readily substituted at the Methine group, methyne (CH) centre in the thiazole ring. Thiazole is electron-withdrawing. Synthesis and biosynthesis Benzothiazoles are typically prepared by treatment of 2-Aminothiophenol, 2-merc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazole
Thiazole (), or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure Thiazoles are members of the azoles, heterocycles that include imidazoles and oxazoles. Thiazole can also be considered a functional group when part of a larger molecule. Being planar thiazoles are characterized by significant pi-electron delocalization and have some degree of aromaticity, more so than the corresponding oxazoles. This aromaticity is evidenced by the 1H NMR chemical shift of the ring protons, which absorb between 7.27 and 8.77 ppm, indicating a strong diamagnetic ring current. The calculated pi-electron density marks C5 as the primary site for electrophilic substitution, and C2-H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallography, crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information. X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences between various materials, especially minerals and alloys. The method has also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomer
In chemistry, tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is a quantum superposition, essentially the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazonium Salt
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent, compound where R is hydrogen, is diazenylium. Structure and general properties Arene derivatives According to X-ray crystallography the linkage is linear in typical diazonium salts. The bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å, which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p''K''a values compared to their unsubstituted counterparts. The p''K''a of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group raises the ionization constant ''K'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Coupling
In organic chemistry, an azo coupling is an organic reaction, reaction between a diazonium compound () and another aromatic compound that produces an azo compound (). In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activating group, activated carbon (usually from an arene, which is called coupling agent), serves as a nucleophile. Classical coupling agents are phenols and naphthols. Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate. Uses of the reaction Aromatic azo compounds tend to be brightly colored due to their extended Conjugated system, conjugated systems. Many are useful dyes (see azo dye). Important azo dyes include methyl red and pigment red 170. Azo printing exploits this reaction as well. In this case, the diazonium ion is degraded by light, leaving a latent image in undegrade ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amines
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
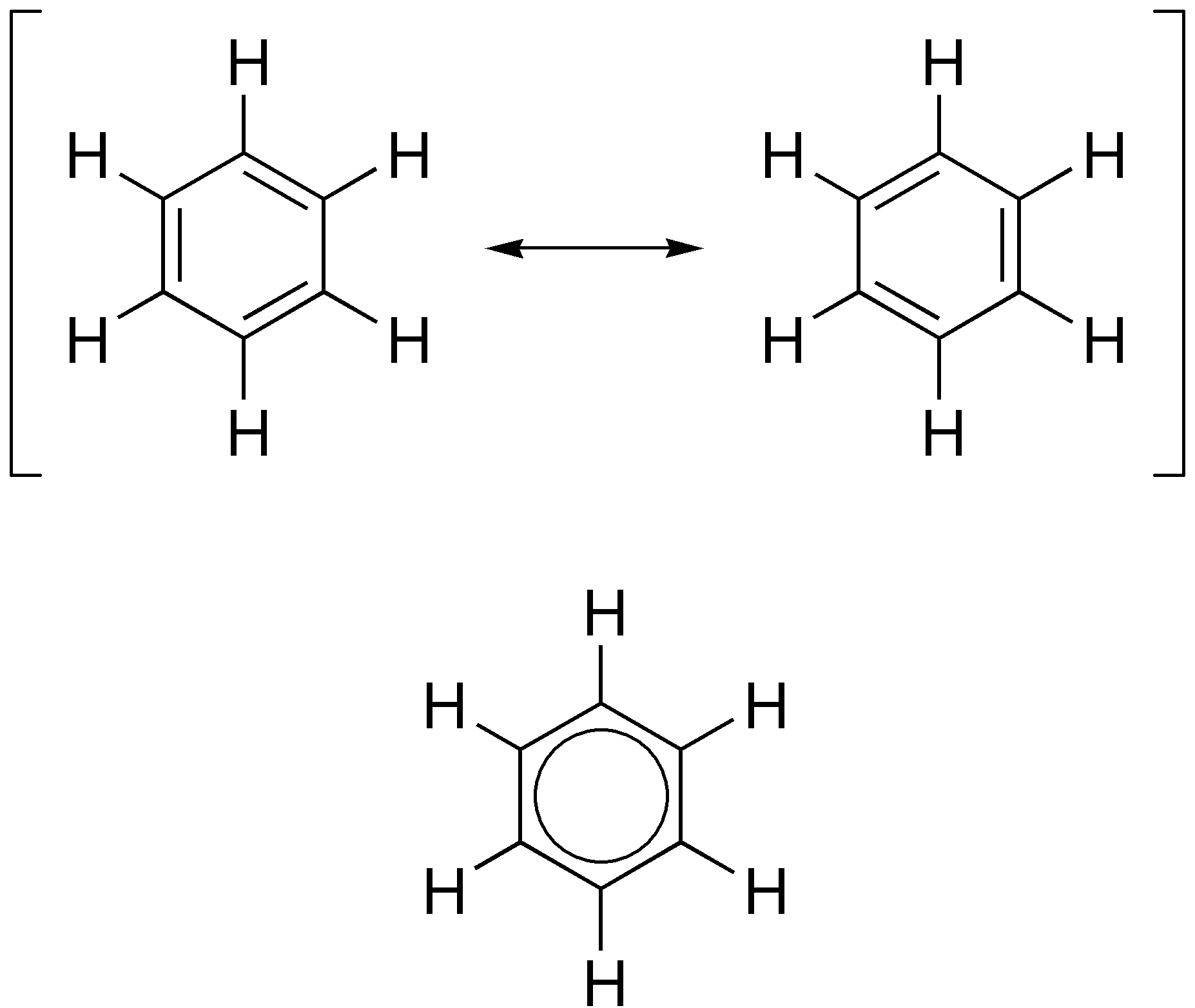
Aromatic Bases
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double- bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). Each bond may be seen as a hybrid of a single bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |