|
α-Isopropylmalate Isomerase
3-Isopropylmalate dehydratase () is an aconitase homologue, which catalyses the isomerisation of isopropylmalate, 2-isopropylmalate to 3-isopropylmalate, via Dehydration reaction, dehydration, in the biosynthesis of leucine. References External links * Lyases {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
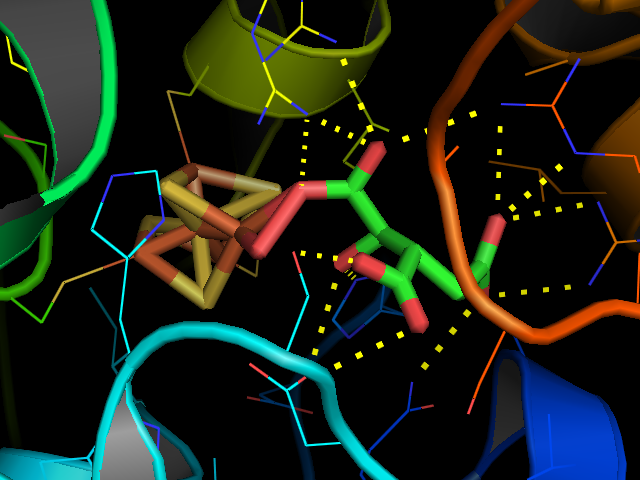
Aconitase
Aconitase (aconitate hydratase; ) is an enzyme that catalyses the stereochemistry, stereo-specific isomerization of citrate to isocitrate via ''cis''-aconitate in the tricarboxylic acid cycle, a non-redox-active process. Image:Citrate wpmp.png, Image:Aconitic acid.svg, Image:isocitric acid.svg, Structure Aconitase has two slightly different structures, depending on whether it is activated or inactivated. In the inactive form, its structure is divided into four domains. Counting from the N-terminus, only the first three of these domains are involved in close interactions with the [3Fe-4S] cluster, but the active site consists of residues from all four domains, including the larger C-terminal domain. The Fe-S cluster and a anion also reside in the active site. When the enzyme is activated, it gains an additional iron atom, creating a [4Fe-4S] cluster. However, the structure of the rest of the enzyme is nearly unchanged; the conserved atoms between the two forms are in es ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |