Aconitase on:
[Wikipedia]
[Google]
[Amazon]
Aconitase (aconitate hydratase; ) is an enzyme that catalyses the stereo-specific
Image:Citrate wpmp.png,
Image:Aconitic acid.svg,
Image:isocitric acid.svg,
isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
of citrate
Citric acid is an organic compound with the formula . It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relati ...
to isocitrate
Isocitric acid is a structural isomer of citric acid. Since citric acid and isocitric acid are structural isomers, they share similar physical and chemical properties. Due to these similar properties, it is difficult to separate the isomers. Salts ...
via ''cis''- aconitate in the tricarboxylic acid cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions that release the energy stored in nutrients through acetyl-CoA oxidation. The e ...
, a non-redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
-active process.
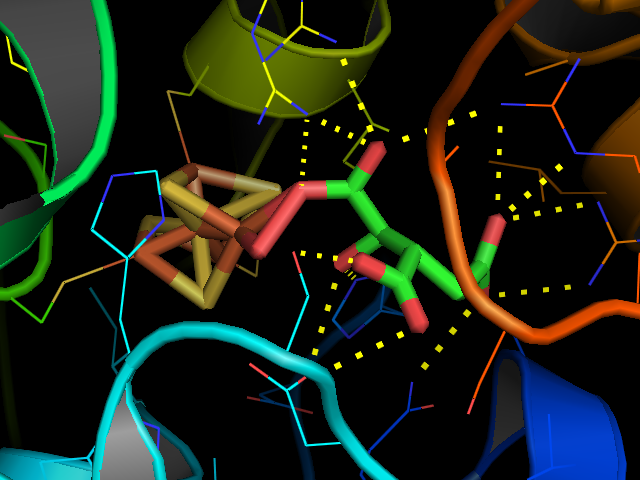
Structure
Aconitase has two slightly different structures, depending on whether it is activated or inactivated. In the inactive form, its structure is divided into four domains. Counting from theN-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
, only the first three of these domains are involved in close interactions with the Fe-4Scluster, but the active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the ''binding s ...
consists of residues from all four domains, including the larger C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When t ...
domain. The Fe-S cluster and a anion also reside in the active site. When the enzyme is activated, it gains an additional iron atom, creating a Fe-4Scluster. However, the structure of the rest of the enzyme is nearly unchanged; the conserved atoms between the two forms are in essentially the same positions, up to a difference of 0.1 angstroms.
Function
In contrast with the majority of iron-sulfur proteins that function as electron carriers, the iron-sulfur cluster of aconitase reacts directly with an enzyme substrate. Aconitase has an active e4S4sup>2+ cluster, which may convert to an inactive e3S4sup>+ form. Threecysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
(Cys) residues have been shown to be ligands of the e4S4centre. In the active state, the labile iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
ion of the e4S4cluster is not coordinated by Cys but by water molecules.
The iron-responsive element-binding protein (IRE-BP) and 3-isopropylmalate dehydratase
3-Isopropylmalate dehydratase () is an aconitase homologue, which catalyses the isomerisation of isopropylmalate, 2-isopropylmalate to 3-isopropylmalate, via Dehydration reaction, dehydration, in the biosynthesis of leucine.
References
Extern ...
(α-isopropylmalate isomerase; ), an enzyme catalysing the second step in the biosynthesis of leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-Car ...
, are known aconitase homologues. Iron regulatory elements (IREs) constitute a family of 28-nucleotide, non-coding, stem-loop structures that regulate iron storage, heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
synthesis and iron uptake. They also participate in ribosome
Ribosomes () are molecular machine, macromolecular machines, found within all cell (biology), cells, that perform Translation (biology), biological protein synthesis (messenger RNA translation). Ribosomes link amino acids together in the order s ...
binding and control the mRNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of Protein biosynthesis, synthesizing a protein.
mRNA is ...
turnover (degradation). The specific regulator protein, the IRE-BP, binds to IREs in both 5' and 3' regions, but only to RNA in the apo form, without the Fe-S cluster. Expression of IRE-BP in cultured cells has revealed that the protein functions either as an active aconitase, when cells are iron-replete, or as an active RNA-binding protein, when cells are iron-depleted. Mutant IRE-BPs, in which any or all of the three Cys residues involved in Fe-S formation are replaced by serine
Serine
(symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − ...
, have no aconitase activity, but retain RNA-binding properties.
Aconitase is inhibited by fluoroacetate, therefore fluoroacetate is poisonous. Fluoroacetate, in the citric acid cycle, is converted to fluorocitrate by citrate synthase. Fluorocitrate competitively inhibits aconitase halting the citric acid cycle. The iron sulfur cluster is highly sensitive to oxidation by superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
.
Mechanism
Aconitase employs a dehydration-hydration mechanism. The catalytic residues involved are His-101 and Ser-642. His-101 protonates the hydroxyl group on C3 of citrate, allowing it to leave as water, and Ser-642 concurrently abstracts the proton on C2, creating a double bond between C2 and C3, and forming the so-called ''cis''-aconitate intermediate (the twocarboxyl group
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ...
s on the double bond are ''cis''). The carbon atom from which the hydrogen is removed is the one that came from oxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes ...
in the previous step of the citric acid cycle, not the one that came from acetyl CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized fo ...
, even though these two carbons are equivalent except that one is "''pro''-R" and the other "''pro''-S" (see Prochirality
In stereochemistry, prochiral molecules are those that can be converted from achiral to Chirality (chemistry), chiral in a single Reaction step, step, such as changing one atom. An achiral species which can be converted to a chiral in two steps i ...
). At this point, the intermediate is rotated 180°. This rotation is referred to as a "flip." Because of this flip, the intermediate is said to move from a "citrate mode" to a "isocitrate mode."
How exactly this flip occurs is debatable. One theory is that, in the rate-limiting step of the mechanism, the ''cis''-aconitate is released from the enzyme, then reattached in the isocitrate mode to complete the reaction. This rate-limiting step ensures that the right stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
, specifically (2R,3S), is formed in the final product. Another hypothesis is that ''cis''-aconitate stays bound to the enzyme while it flips from the citrate to the isocitrate mode.
In either case, flipping ''cis''-aconitate allows the dehydration and hydration steps to occur on opposite faces of the intermediate. Aconitase catalyzes ''trans'' elimination/addition of water, and the flip guarantees that the correct stereochemistry is formed in the product. To complete the reaction, the serine and histidine residues reverse their original catalytic actions: the histidine, now basic, abstracts a proton from water, priming it as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
to attack at C2, and the protonated serine is deprotonated by the ''cis''-aconitate double bond to complete the hydration, producing isocitrate.

Family members
Aconitases are expressed in bacteria to humans. In citrus fruits, a reduction of the activity of the mitochondrial aconitases likely leads to the buildup of citric acid, which is then stored invacuole
A vacuole () is a membrane-bound organelle which is present in Plant cell, plant and Fungus, fungal Cell (biology), cells and some protist, animal, and bacterial cells. Vacuoles are essentially enclosed compartments which are filled with water ...
s. As the fruit matures, citric acid is returned back to the cytosol where an increase in cytosolic aconitase activity reduces its levels in the fruit. Humans express the following two aconitase isozyme
In biochemistry, isozymes (also known as isoenzymes or more generally as multiple forms of enzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. Isozymes usually have different kinetic parameters (e.g. di ...
s:
Interactive pathway map
References
Further reading
*External links
* * - the Aconitase structure in interactive 3D {{Portal bar, Biology, border=no EC 4.2.1 Iron–sulfur proteins Moonlighting proteins