
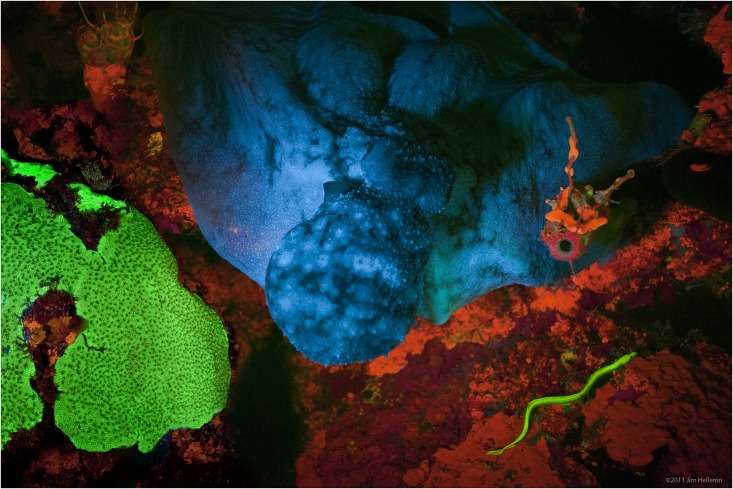

Fluorescence is one of two kinds of
photoluminescence
Photoluminescence (abbreviated as PL) is light emission from any form of matter after the absorption of photons (electromagnetic radiation). It is one of many forms of luminescence (light emission) and is initiated by photoexcitation (i.e. phot ...
, the emission of
light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
by a substance that has absorbed light or other
electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
. When exposed to
ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission,
phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluor ...
. Phosphorescent materials continue to emit light for some time after the radiation stops.
This difference in duration is a result of quantum spin effects.
Fluorescence occurs when a
photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
from incoming radiation is absorbed by a
molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
, exciting it to a higher
energy level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
, followed by the emission of light as the molecule returns to a lower energy state. The emitted light may have a longer
wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
and, therefore, a lower
photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
than the absorbed radiation. For example, the absorbed radiation could be in the
ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
region of the
electromagnetic spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high ...
(invisible to the human eye), while the emitted light is in the
visible region. This gives the fluorescent substance a distinct
color
Color (or colour in English in the Commonwealth of Nations, Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is the visual perception based on the electromagnetic spectrum. Though co ...
, best seen when exposed to
UV light, making it appear to glow in the dark. However, any light with a shorter wavelength may cause a material to fluoresce at a longer wavelength. Fluorescent materials may also be excited by certain wavelengths of visible light, which can mask the glow, yet their colors may appear bright and intensified. Other fluorescent materials emit their light in the infrared or even the ultraviolet regions of the spectrum.
Fluorescence has many practical applications, including
mineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical mineralogy, optical) properties of minerals and mineralized artifact (archaeology), artifacts. Specific s ...
,
gemology
Gemology or gemmology is the science dealing with natural and artificial gemstone materials. It is a specific interdisciplinary branch of mineralogy. Some jewellery, jewelers (and many non-jewelers) are academically trained gemologists and are qua ...
,
medicine
Medicine is the science and Praxis (process), practice of caring for patients, managing the Medical diagnosis, diagnosis, prognosis, Preventive medicine, prevention, therapy, treatment, Palliative care, palliation of their injury or disease, ...
, chemical sensors (
fluorescence spectroscopy
Fluorescence spectroscopy (also known as fluorimetry or spectrofluorometry) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electro ...
),
fluorescent labelling
In molecular biology and biotechnology, a fluorescent tag, also known as a fluorescent label or fluorescent probe, is a molecule that is attached chemically to aid in the detection of a biomolecule such as a protein, antibody, or amino acid. Gener ...
,
dye
Juan de Guillebon, better known by his stage name DyE, is a French musician. He is known for the music video of the single "Fantasy
Fantasy is a genre of speculative fiction that involves supernatural or Magic (supernatural), magical ele ...
s, biological detectors, cosmic-ray detection,
vacuum fluorescent display
A vacuum fluorescent display (VFD) is a display device once commonly used on consumer electronics equipment such as video cassette recorders, car audio, car radios, and microwave ovens.
A VFD operates on the principle of cathodoluminescence, ...
s, and
cathode-ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms on an oscilloscope, a ...
s. Its most common everyday application is in (
gas-discharge)
fluorescent lamp
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, to produce ultraviolet and make a phosphor ...
s and
LED lamps
An LED lamp or LED light is an electric light that produces light using light-emitting diodes (LEDs). LED lamps are significantly more Electrical efficiency, energy-efficient than equivalent Incandescent light bulb, incandescent lamps and f ...
, where fluorescent coatings convert UV or blue light into longer wavelengths, resulting in
white light, which can appear indistinguishable from that of the traditional but energy-inefficient
incandescent lamp
An incandescent light bulb, also known as an incandescent lamp or incandescent light globe, is an electric light that produces illumination by Joule heating a filament until it glows. The filament is enclosed in a glass bulb that is eith ...
.
Fluorescence also occurs frequently in nature, appearing in some minerals and many biological forms across all kingdoms of life. The latter is often referred to as ''
biofluorescence
Biofluorescence is fluorescence exhibited by a living organism: part of the organism absorbs light or other radiation at one wavelength and emits visible light at another, usually longer. The absorbed radiation is often blue or ultraviolet, while t ...
'', indicating that the
fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with se ...
is part of or derived from a living organism (rather than an inorganic
dye
Juan de Guillebon, better known by his stage name DyE, is a French musician. He is known for the music video of the single "Fantasy
Fantasy is a genre of speculative fiction that involves supernatural or Magic (supernatural), magical ele ...
or
stain
A stain is a discoloration that can be clearly distinguished from the surface, material, or medium it is found upon. They are caused by the chemical or physical interaction of two dissimilar materials. Accidental staining may make materials app ...
). However, since fluorescence results from a specific chemical property that can often be synthesized artificially, it is generally sufficient to describe the substance itself as ''fluorescent''.
History
Fluorescence was observed long before it was named and understood.
[
]
An early observation of fluorescence was known to the Aztecs
[ and described in 1560 by Bernardino de Sahagún and in 1565 by Nicolás Monardes in the ]infusion
Infusion is the process of extracting chemical compounds or flavors from plant material in a solvent such as water, oil or alcohol, by allowing the material to remain suspended in the solvent over time (a process often called steeping). An inf ...
known as '' lignum nephriticum'' (Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
for "kidney wood"). It was derived from the wood of two tree species, '' Pterocarpus indicus'' and '' Eysenhardtia polystachya''.[
][
]
The chemical compound responsible for this fluorescence is matlaline, which is the oxidation product of one of the flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.
Chemically, flavonoids ...
s found in this wood.[
In 1819, E.D. Clarke
and in 1822 ]René Just Haüy
René Just Haüy () FRS MWS FRSE (28 February 1743 – 1 June 1822) was a French priest and mineralogist, commonly styled the Abbé Haüy after he was made an honorary canon of Notre-Dame de Paris, Notre Dame. Due to his innovative work on cryst ...
[
]
described some varieties of fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs scal ...
s that had a different color depending on whether the light was reflected or (apparently) transmitted. Haüy incorrectly viewed the effect as light scattering similar to opalescence.[ In 1833 Sir David Brewster described a similar effect in ]chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy ...
which he also considered a form of opalescence.
Sir John Herschel studied quinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg ...
in 1845 and came to a different incorrect conclusion.[
In 1842, A.E. Becquerel observed that calcium sulfide emits light after being exposed to solar ]ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
, making him the first to state that the emitted light is of longer wavelength than the incident light. While his observation of photoluminescence
Photoluminescence (abbreviated as PL) is light emission from any form of matter after the absorption of photons (electromagnetic radiation). It is one of many forms of luminescence (light emission) and is initiated by photoexcitation (i.e. phot ...
was similar to that described 10 years later by Stokes, who observed a fluorescence of a solution of quinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg ...
, the phenomenon that Becquerel described with calcium sulfide is now called phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluor ...
.[
In his 1852 paper on the "Refrangibility" (]wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
change) of light, George Gabriel Stokes
Sir George Gabriel Stokes, 1st Baronet, (; 13 August 1819 – 1 February 1903) was an Irish mathematician and physicist. Born in County Sligo, Ireland, Stokes spent his entire career at the University of Cambridge, where he served as the Lucasi ...
described the ability of fluorspar, uranium glass and many other substances to change invisible light beyond the violet end of the visible spectrum into visible light. He named this phenomenon ''fluorescence''[
: "I am almost inclined to coin a word, and call the appearance ''fluorescence'', from fluor-spar .e., fluorite as the analogous term ''opalescence'' is derived from the name of a mineral."][
]
Neither Becquerel nor Stokes understood one key aspect of photoluminescence: the critical difference from incandescence
Thermal radiation is electromagnetic radiation emitted by the thermal motion of particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation. The emission of energy arises from a combination of electron ...
, the emission of light by heated material. To distinguish it from incandescence, in the late 1800s, Gustav Wiedemann proposed the term luminescence
Luminescence is a spontaneous emission of radiation from an electronically or vibrationally excited species not in thermal equilibrium with its environment. A luminescent object emits ''cold light'' in contrast to incandescence, where an obje ...
to designate any emission of light more intense than expected from the source's temperature.[
Advances in ]spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectro ...
and quantum electronics
Quantum optics is a branch of atomic, molecular, and optical physics and quantum chemistry that studies the behavior of photons (individual quanta of light). It includes the study of the particle-like properties of photons and their interaction w ...
between the 1950s and 1970s provided a way to distinguish between the three different mechanisms that produce the light, as well as narrowing down the typical timescales those mechanisms take to decay after absorption. In modern science, this distinction became important because some items, such as lasers, required the fastest decay times, which typically occur in the nanosecond (billionth of a second) range. In physics, this first mechanism was termed "fluorescence" or "singlet emission", and is common in many laser mediums such as ruby. Other fluorescent materials were discovered to have much longer decay times, because some of the atoms would change their spin to a triplet state
In quantum mechanics, a triplet state, or spin triplet, is the quantum state of an object such as an electron, atom, or molecule, having a quantum spin ''S'' = 1. It has three allowed values of the spin's projection along a given axis ''m''S = � ...
, thus would glow brightly with fluorescence under excitation but produce a dimmer afterglow for a short time after the excitation was removed, which became labeled "phosphorescence" or "triplet phosphorescence". The typical decay times ranged from a few microseconds to one second, which are still fast enough by human-eye standards to be colloquially referred to as fluorescent. Common examples include fluorescent lamps, organic dyes, and even fluorspar. Longer emitters, commonly referred to as glow-in-the-dark substances, ranged from one second to many hours, and this mechanism was called persistent phosphorescence or persistent luminescence, to distinguish it from the other two mechanisms.
Physical principles
Mechanism
Fluorescence occurs when an excited molecule, atom, or nanostructure
A nanostructure is a structure of intermediate size between microscopic and molecular structures. Nanostructural detail is microstructure at nanoscale.
In describing nanostructures, it is necessary to differentiate between the number of dimen ...
, relaxes to a lower energy state (usually the ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state ...
) through emission of a photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
without a change in electron spin
Spin is an intrinsic form of angular momentum carried by elementary particles, and thus by composite particles such as hadrons, atomic nuclei, and atoms. Spin is quantized, and accurate models for the interaction with spin require relativistic ...
. When the initial and final states have different multiplicity (spin), the phenomenon is termed phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluor ...
.
When a molecule in its ground state (called S0) is photoexcited it may end up in any one of a number of excited states (S1, S2, S3,...). These higher excited states are different vibrational levels, populated in proportion to their overlap with the ground state according to the Franck-Condon principle.singlet state
In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s=0. A ...
s.triplet state
In quantum mechanics, a triplet state, or spin triplet, is the quantum state of an object such as an electron, atom, or molecule, having a quantum spin ''S'' = 1. It has three allowed values of the spin's projection along a given axis ''m''S = � ...
T1. Decay from T1 to S0 is typically slower and less intense and is called phosphorescence.heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
. Thus the fluorescence energy is typically less than the photoexcitation energy.internal conversion
Internal conversion is an atomic decay process where an excited nucleus interacts electromagnetically with one of the orbital electrons of an atom. This causes the electron to be emitted (ejected) from the atom. Thus, in internal conversion (o ...
, intersystem crossing
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.
Excited singlet and triplet states
When an electron in a molecule with a singlet grou ...
to the triplet state, and energy transfer to another molecule. An example of energy transfer is Förster resonance energy transfer
Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores). ...
. Relaxation from an excited state can also occur through collisional quenching
In materials science, quenching is the rapid cooling of a workpiece in water, gas, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, suc ...
, a process where a molecule (the quencher) collides with the fluorescent molecule during its excited state lifetime. Molecular oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
(O2) is an extremely efficient quencher of fluorescence because of its unusual triplet ground state.
Quantum yield
The fluorescence quantum yield
In particle physics, the quantum yield (denoted ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
\Phi(\lambda)=\frac
Applications
Fluorescence spectroscopy
The fluorescence ...
gives the efficiency of the fluorescence process. It is defined as the ratio of the number of photons emitted to the number of photons absorbed.[
]
:
The maximum possible fluorescence quantum yield is 1.0 (100%); each photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
absorbed results in a photon emitted. Compounds with quantum yields of 0.10 are still considered quite fluorescent. Another way to define the quantum yield of fluorescence is by the rate of excited state decay:
:
where is the rate constant of spontaneous emission
Spontaneous emission is the process in which a Quantum mechanics, quantum mechanical system (such as a molecule, an atom or a subatomic particle) transits from an excited state, excited energy state to a lower energy state (e.g., its ground state ...
of radiation and
:
is the sum of all rates of excited state decay. Other rates of excited state decay are caused by mechanisms other than photon emission and are, therefore, often called "non-radiative rates", which can include:
* dynamic collisional quenching
* near-field dipole–dipole interaction (or resonance energy transfer)
* internal conversion
* intersystem crossing
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.
Excited singlet and triplet states
When an electron in a molecule with a singlet grou ...
Thus, if the rate of any pathway changes, both the excited state lifetime and the fluorescence quantum yield will be affected.
Fluorescence quantum yields are measured by comparison to a standard. The quinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg ...
salt ''quinine sulfate'' in a sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
solution was regarded as the most common fluorescence standard,
however, a recent study revealed that the fluorescence quantum yield of this solution is strongly affected by the temperature, and should no longer be used as the standard solution. The quinine in 0.1 M perchloric acid () shows no temperature dependence up to 45 °C, therefore it can be considered as a reliable standard solution.
Lifetime
 The fluorescence lifetime refers to the average time the molecule stays in its excited state before emitting a photon. Fluorescence typically follows first-order kinetics:
:
where
The fluorescence lifetime refers to the average time the molecule stays in its excited state before emitting a photon. Fluorescence typically follows first-order kinetics:
:
where


 Fluorescence is one of two kinds of
Fluorescence is one of two kinds of  The fluorescence lifetime refers to the average time the molecule stays in its excited state before emitting a photon. Fluorescence typically follows first-order kinetics:
:
where
The fluorescence lifetime refers to the average time the molecule stays in its excited state before emitting a photon. Fluorescence typically follows first-order kinetics:
:
where


 Fluorescence is one of two kinds of
Fluorescence is one of two kinds of