|
Potassium Oxide
Potassium oxide ( K O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertilizers and cements, are assayed assuming the percent composition that would be equivalent to K2O. Production Potassium oxide is produced from the reaction of oxygen and potassium; this reaction affords potassium peroxide, K2O2. Treatment of the peroxide with potassium produces the oxide: : K2O2 + 2 K -> 2 K2O Alternatively and more conveniently, K2O is synthesized by heating potassium nitrate with metallic potassium: :2KNO3 + 10K -> 6K2O + N2 (^) Other possibility is to heat potassium peroxide at 500 °C which decomposes at that temperature giving pure potassium oxide and oxygen. :2K2O2 -> 2K2O + O2 (^) Potassium hydroxide cannot be further dehydrated to the oxide but it can react with molten potassium to produce it, releasing hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potash
Potash () includes various mined and manufactured salts that contain potassium in water-soluble form.Potash USGS 2008 Minerals Yearbook The name derives from ''pot ash'', plant ashes or wood ash soaked in water in a pot, the primary means of manufacturing potash before the Industrial Era. The word '' potassium'' is derived from ''potash''. Potash is produced worldwide in amounts exceeding 90 million s (40 million tonnes [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthocl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Fluoride
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities. Chemical structure The compound crystallizes in a cubic motif called the fluorite structure. Ca2+ centres are eight-coordinate, being centered in a cube of eight F− centres. Each F− centre is coordinated to four Ca2+ centres in the shape of a tetrahedron. Although perfectly packed crystalline samples are colorless, the mineral is often deeply colored due to the presence of F-centers. The same crystal structure is found in numerous ionic compounds with formula AB2, such as CeO2, cubic ZrO2, UO2, ThO2, and PuO2. In the corresponding anti-structure, called the antifluorite structure, anions and cations are swapped, such as Be2C. Gas phase The gas phase is noteworthy for failing the predictions of VSEPR theory; the molecule is no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
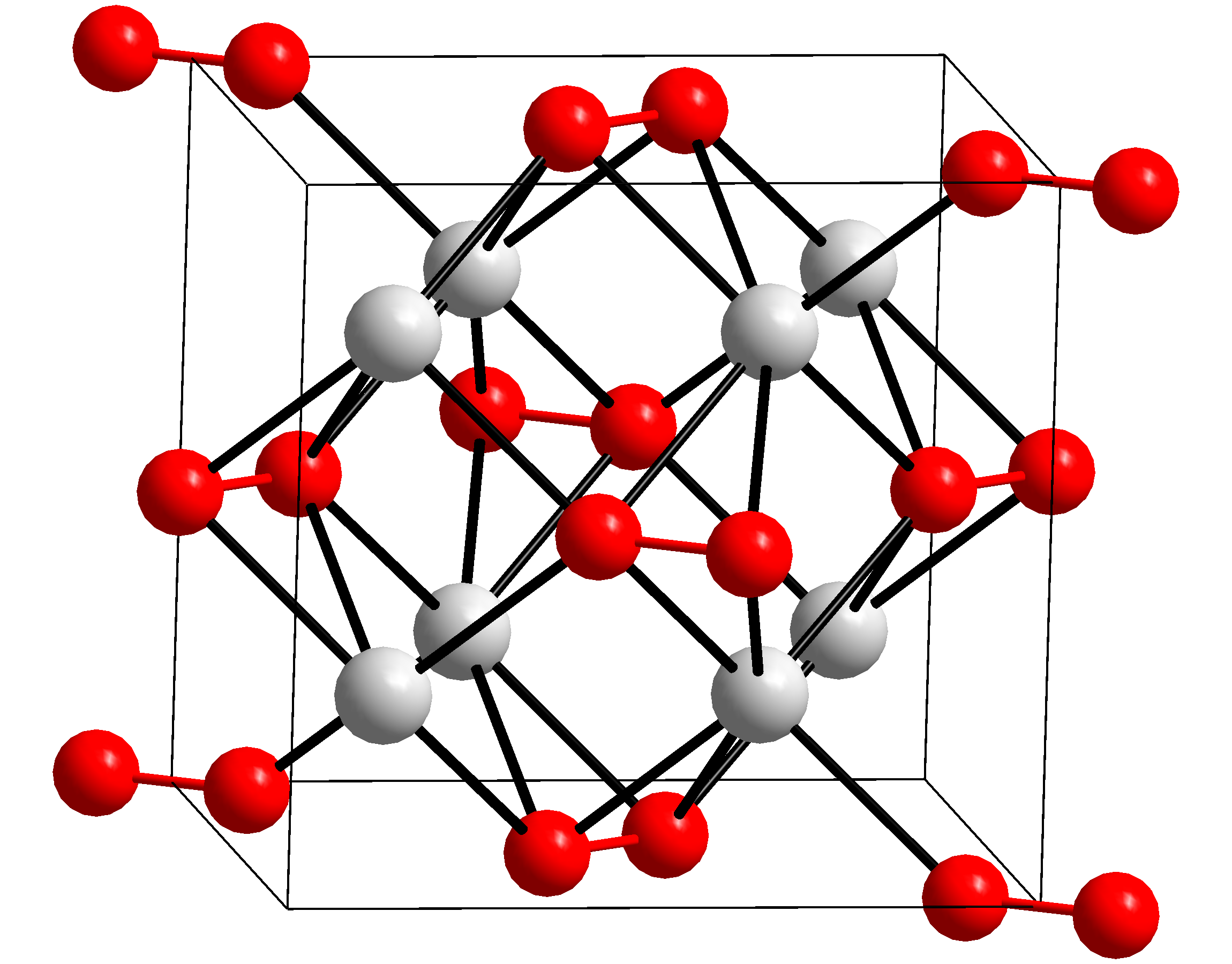
Fluorite Structure
In solid state chemistry, the fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure. Many compounds, notably the common mineral fluorite (CaF2), adopt this structure. Many compounds with formula M2X have an antifluorite structure. In these the locations of the anions and cations are reversed relative to fluorite (an anti-structure); the anions occupy the FCC regular sites whereas the cations occupy the tetrahedral interstitial sites. For example, Magnesium silicide, Mg2Si, has a lattice parameter of 6.338 Å with magnesium cations occupying the tetrahedral interstitial sites, in which each silicide anion is surrounded by eight magnesium cations and each magnesium cation is surrounded by four silicide anions in a tetrahedral fashion. File:Fluorite Structure.jpg, The fluorite structure of calcium fluoride CaF2. File:An ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Nitrate
Potassium nitrate is a chemical compound with the chemical formula . This alkali metal nitrate salt is also known as Indian saltpetre (large deposits of which were historically mined in India). It is an ionic salt of potassium ions K+ and nitrate ions NO3−, and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or ''nitre'' in the UK). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpeter (or ''saltpetre'' in the UK). Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of gunpowder (black powder). In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color. Etymology Potassium nitrate, because of its early and global use and production, has many names. Hebrew and Egyptian words for it had the consonants n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Peroxide
Potassium peroxide is an inorganic compound with the molecular formula K2O2. It is formed as potassium reacts with oxygen in the air, along with potassium oxide (K2O) and potassium superoxide (KO2). Potassium peroxide reacts with water to form potassium hydroxide and oxygen: : 2K2O2 + 2H2O -> 4KOH + O2 (^) Properties Potassium peroxide is a highly reactive, oxidizing white to yellowish solid which, while not flammable itself, reacts violently with flammable materials. It decomposes violently on contact with water Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living .... The standard enthalpy of formation of potassium peroxide is ΔH f 0 = −496 kJ/mol. Usage Potassium Peroxide is used as an oxidizing agent and bleach (due to the peroxide), and to purify air. References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the autoionization equilibrium, bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |