Wagner–Meerwein Rearrangement on:
[Wikipedia]
[Google]
[Amazon]
A Wagner–Meerwein rearrangement is a class of  The story of the rearrangement reveals that many scientists were puzzled with this and related reactions and its close relationship to the discovery of
The story of the rearrangement reveals that many scientists were puzzled with this and related reactions and its close relationship to the discovery of  Currently, there are works relating to the use of skeletal rearrangement in the synthesis of bridged azaheterocycles. These data are summarized in
:
Currently, there are works relating to the use of skeletal rearrangement in the synthesis of bridged azaheterocycles. These data are summarized in
: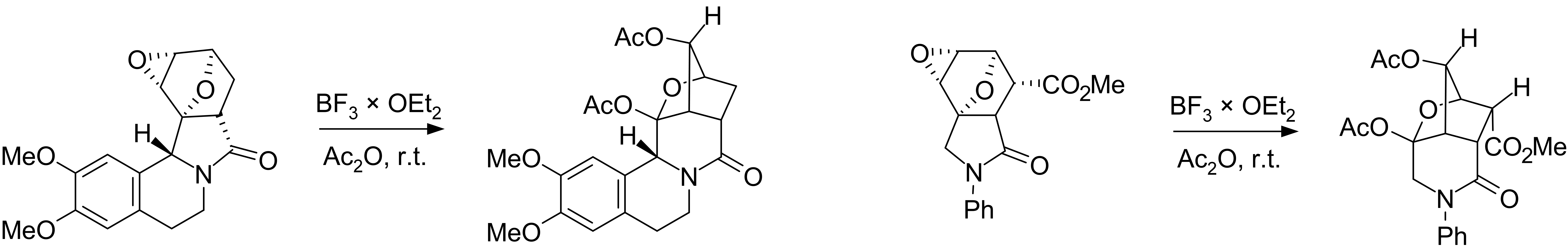 Plausible mechanisms of the Wagner–Meerwein rearrangement of diepoxyisoindoles:
Plausible mechanisms of the Wagner–Meerwein rearrangement of diepoxyisoindoles:
 The related Nametkin rearrangement, named after
The related Nametkin rearrangement, named after
carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Frank C. Whitmore, Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atom ...
reaction
Reaction may refer to a process or to a response to an action, event, or exposure.
Physics and chemistry
*Chemical reaction
*Nuclear reaction
*Reaction (physics), as defined by Newton's third law
* Chain reaction (disambiguation)
Biology and ...
s in which a hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
group migrates from one carbon to a neighboring carbon.
They can be described as cationic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
,2 sigmatropic rearrangements, proceeding suprafacially and with stereochemical
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
retention. As such, a Wagner–Meerwein shift is a thermally allowed pericyclic
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overl ...
process with the Woodward-Hoffmann symbol sub>ω0s + σ2s They are usually facile, and in many cases, they can take place at temperatures as low as –120 °C. The reaction is named after the Russian chemist Yegor Yegorovich Vagner
Yegor Yegorovich Wagner (Russian Егор Егорович Вагнер, sometimes Georg Wagner or Egor Vagner; 9 December 1849 – 27 November 1903) was a Russian organic chemist, famous for the discovery of the "Wagner reaction", named after him ...
; he had German origin and published in German journals as Georg Wagner; and Hans Meerwein
Hans Meerwein (May 20, 1879 in Hamburg, Germany – October 24, 1965 in Marburg, Germany) was a German chemist.
Several reactions and reagents bear his name, most notably the Meerwein–Ponndorf–Verley reduction, the Wagner–Meerwein rearr ...
.
Several reviews have been published.
The rearrangement was first discovered in bicyclic
A bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring ...
terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predomi ...
s for example the conversion of isoborneol
Isoborneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an ''Endo-exo isomerism, exo'' position. The endo diastereomer is called borneol. Being chiral, isoborneol exists as enantiomers. ...
to camphene
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As with other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as tu ...
:
carbocations
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
as intermediates.
In a simple demonstration reaction of 1,4-dimethoxybenzene with either 2-methyl-2-butanol
''tert''-Amyl alcohol (TAA) or 2-methylbutan-2-ol (2M2B), is a branched pentanol.
Historically, TAA has been used as an anesthetic and more recently as a recreational drug. TAA is mostly a positive allosteric modulator for GABAA receptors in th ...
or 3-methyl-2-butanol in sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
and acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
yields the same disubstituted product, the latter via a hydride shift of the cationic intermediate:
: Plausible mechanisms of the Wagner–Meerwein rearrangement of diepoxyisoindoles:
Plausible mechanisms of the Wagner–Meerwein rearrangement of diepoxyisoindoles:
Sergey Namyotkin
Sergey Semyonovich Nametkin (; – 5 August 1950) was a Soviet and Russian organic chemist, a prominent researcher in terpene chemistry, the cracking of petrochemicals, and rearrangement of camphenes. Academician of the USSR Academy of Scien ...
, involves the rearrangement of methyl groups in certain terpenes. In some cases the reaction type is also called a retropinacol rearrangement (see pinacol rearrangement
The pinacol–pinacolone rearrangement is a method for converting a diol, 1,2-diol to a carbonyl compound in organic chemistry. The 1,2-rearrangement takes place under acidic conditions. The name of the rearrangement reaction comes from the rearra ...
).
References
{{DEFAULTSORT:Wagner-Meerwein rearrangement Rearrangement reactions Name reactions