|
Sigmatropic
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one sigma bond, σ-bond is changed to another σ-bond in an uncatalyzed intramolecular reaction. The name ''sigmatropic'' is the result of a compound word, compounding of the long-established sigma designation from single carbon–carbon bonds and the Greek word ''tropos'', meaning turn. In this type of rearrangement reaction, a substituent moves from one part of a pi-bond, π-bonded system to another part in an intramolecular reaction with simultaneous rearrangement of the π system. True sigmatropic reactions are usually uncatalyzed, although Lewis acid catalysis is possible. Sigmatropic reactions often have transition-metal catalysts that form intermediates in analogous reactions. The most well-known of the sigmatropic rearrangements are the [3,3] Cope rearrangement, Claisen rearrangement, Carroll rearrangement, and the Fischer indole synthesis. Overview of sigmatropic shifts Woodward� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Woodward–Hoffmann Rules
The Woodward–Hoffmann rules (or the pericyclic selection rules), devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules used to rationalize or predict certain aspects of the stereochemistry and activation energy of pericyclic reactions, an important class of reactions in organic chemistry. The rules are best understood in terms of the concept of ''the conservation of orbital symmetry'' using ''orbital correlation diagrams'' (see Section 3 below). The Woodward–Hoffmann rules are a consequence of the changes in electronic structure that occur during a pericyclic reaction and are predicated on the phasing of the interacting molecular orbitals. They are applicable to all classes of pericyclic reactions (and their microscopic reverse 'retro' processes), including (1) electrocyclizations, (2) cycloadditions, (3) sigmatropic reactions, (4) group transfer reactions, (5) ene reactions, (6) cheletropic reactions, and (7) dyotropic reactions. Due to their elegance, simp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
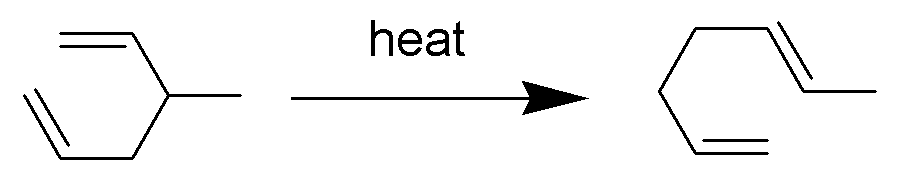
Cope Rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the ,3sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope and Elizabeth Hardy. For example, 3-methyl-hexa-1,5-diene heated to 300 °C yields hepta-1,5-diene. The Cope rearrangement causes the fluxional states of the molecules in the bullvalene family. Mechanism The Cope rearrangement is the prototypical example of a concerted sigmatropic rearrangement. It is classified as a ,3sigmatropic rearrangement with the Woodward–Hoffmann symbol π2s+σ2s+π2s">sub>π2s+σ2s+π2sand is therefore thermally allowed. It is sometimes useful to think of it as going through a transition state energetically and structurally equivalent to a diradical, although the diradical is not usually a true intermediate (potential energy minimum). The chair transition state illustrated here is preferred in open-chain systems (as shown by the Doering-Roth experiments). However, conformationally co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Rearrangement
The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a ,3sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation (ΔΔHf = -327kcalmol−1). Mechanism The Claisen rearrangement is an exothermic, concerted (bond cleavage and recombination) pericyclic reaction. Woodward–Hoffmann rules show a suprafacial, stereospecific reaction pathway. The kinetics are of the first order and the whole transformation proceeds through a highly ordered cyclic transition state and is intramolecular. Crossover experiments eliminate the possibility of the rearrangement occurring via an intermolecular reaction mechanism and are consistent with an intramolecular process. There are substantial solvent effects observed in the Claisen rearrangement, where polar solvents tend to accelerate the reaction to a gre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antarafacial And Suprafacial
Antarafacial ( Woodward-Hoffmann symbol a) and suprafacial (s) are two topological concepts in organic chemistry describing the relationship between two simultaneous chemical bond making and/or bond breaking processes in or around a reaction center. The reaction center can be a p- or sp''n-''orbital (Woodward-Hoffmann symbol ω), a conjugated system (π) or even a sigma bond (σ). * The relationship is ''antarafacial'' when opposite faces of the π system or isolated orbital are involved in the process (think ''anti''). For a σ bond, it corresponds to involvement of one "interior" lobe and one "exterior" lobe of the bond. * The relationship is ''suprafacial'' when the same face of the π system or isolated orbital are involved in the process (think ''syn''). For a σ bond, it corresponds to involvement of two "interior" lobes or two "exterior" lobes of the bond. The components of all pericyclic reactions, including sigmatropic reactions and cycloadditions, and electrocycl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer Indole Synthesis
The Fischer indole synthesis is a chemical reaction that produces the aromatic Heterocyclic compound, heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer. Today migraine, antimigraine drugs of the triptan class are often synthesized by this method. This reaction can be catalyzed by Brønsted acids such as HCl, sulfuric acid, H2SO4, polyphosphoric acid and p-toluenesulfonic acid or Lewis acids such as boron trifluoride, zinc chloride, iron chloride, and aluminium chloride. Several reviews have been published. Reaction mechanism The reaction of a (substituted) phenylhydrazine with a carbonyl (aldehyde or ketone) initially forms a phenylhydrazone which isomerization, isomerizes to the respective enamine (or 'ene-hydrazine'). After protonation, a cyclic sigmatropic rearrangement, [3,3]-sigmatropic rearrangement occurs producing an imine. The resulting imine forms a cyclic amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pericyclic Reaction
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap in a continuous cycle at the transition state. Pericyclic reactions stand in contrast to ''linear reactions'', encompassing most organic transformations and proceeding through an acyclic transition state, on the one hand and '' coarctate reactions'', which proceed through a doubly cyclic, concerted transition state on the other hand. Pericyclic reactions are usually rearrangement or addition reactions. The major classes of pericyclic reactions are given in the table below (the three most important classes are shown in bold). Ene reactions and cheletropic reactions are often classed as group transfer reactions and cycloadditions/cycloeliminations, respectively, while dyotropic reactions and group transfer reactions (if ene reactions are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suprafacial Antarafacial -1,5-
Antarafacial ( Woodward-Hoffmann symbol a) and suprafacial (s) are two topological concepts in organic chemistry describing the relationship between two simultaneous chemical bond making and/or bond breaking processes in or around a reaction center. The reaction center can be a p- or sp''n-''orbital (Woodward-Hoffmann symbol ω), a conjugated system (π) or even a sigma bond (σ). * The relationship is ''antarafacial'' when opposite faces of the π system or isolated orbital are involved in the process (think ''anti''). For a σ bond, it corresponds to involvement of one "interior" lobe and one "exterior" lobe of the bond. * The relationship is ''suprafacial'' when the same face of the π system or isolated orbital are involved in the process (think ''syn''). For a σ bond, it corresponds to involvement of two "interior" lobes or two "exterior" lobes of the bond. The components of all pericyclic reactions, including sigmatropic reactions and cycloadditions, and electrocyclizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Burns Woodward
Robert Burns Woodward (April 10, 1917 – July 8, 1979) was an American organic chemist. He is considered by many to be the most preeminent synthetic organic chemist of the twentieth century, having made many key contributions to the subject, especially in the synthesis of complex natural products and the determination of their molecular structure. He also worked closely with Roald Hoffmann on theoretical studies of chemical reactions. He was awarded the Nobel Prize in Chemistry in 1965. Early life and education Woodward was born in Boston, Massachusetts, on April 10, 1917. He was the son of Margaret Burns (an immigrant from Scotland who claimed to be a descendant of the poet, Robert Burns) and her husband, Arthur Chester Woodward, himself the son of Roxbury apothecary, Harlow Elliot Woodward. His father was one of the many victims of the 1918 influenza pandemic of 1918. From a very early age, Woodward was attracted to and engaged in private study of chemistry while he at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Woodward Hoffmann Order Nomenclature Bond Break
A woodward is a warden of a wood. Woodward may also refer to: Places ;United States * Woodward, Iowa * Woodward, Oklahoma * Woodward, Pennsylvania, a census-designated place * Woodward Avenue, a street in Tallahassee, Florida, which bisects the campus of Florida State University * Woodward Avenue, a Michigan state highway * Woodward Corridor, a neighborhood in Detroit, Michigan * Woodward County, Oklahoma * Woodward Park (other), multiple places * Woodward Pond, a man-made pond in Bowie, Maryland * Woodward Township, Pennsylvania (other), multiple places People * Woodward (surname) * Frank Lee Woodward (1871–1952), English educationist, Pali scholar, author and theosophist Businesses * Woodward, Inc., American maker of energy devices * Woodward & Lothrop, American department store chain * Woodward Iron Company, in Birmingham (Woodward) Alabama * Woodward's, Canadian department store chain ** The Woodward's building in Vancouver, British Columbia Educati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereochemistry Rentention Inversion
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in structural formula (the three-dimensional orientations of their atoms in space). For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question (dynamic stereochemistr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term ''atomic orbital'' may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital. Each orbital in an atom is characterized by a set of values of the three quantum numbers , , and , which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component ( magnetic quantum number). Alternative to the magnetic quantum number, the orbitals are often labeled by the associated harmonic polynomials (e.g., ''xy'', ). Each such orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simple names s orbital ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |