Uranium hexafluoride on:
[Wikipedia]
[Google]
[Amazon]
Uranium hexafluoride, sometimes called hex, is the
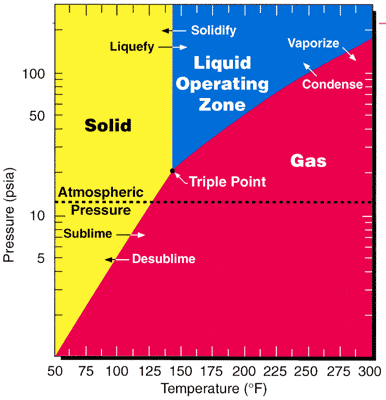 As one of the most volatile compounds of uranium, uranium hexafluoride is relatively convenient to process and is used in both of the main uranium enrichment methods, namely gaseous diffusion and the gas centrifuge method. Since the
As one of the most volatile compounds of uranium, uranium hexafluoride is relatively convenient to process and is used in both of the main uranium enrichment methods, namely gaseous diffusion and the gas centrifuge method. Since the
Uranium
, in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Hrsg.): ''The Chemistry of the Actinide and Transactinide Elements'', Springer, Dordrecht 2006; , p. 253–698; (p. 530–531, 557–564). * US-Patent 2535572
26. December 1950. * US-Patent 5723837
3. March 1998.
Uranium Hexafluoride (UF6) – Physical and chemical properties of UF6, and its use in uranium processing – Uranium Hexafluoride and Its Properties
at WebElements
Import of Western depleted uranium hexafluoride (uranium tails) to Russia
ead link 30 June 2017 {{Actinide halides Actinide halides Hexafluorides Nuclear materials Octahedral compounds Uranium(VI) compounds
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the formula . Uranium hexafluoride is a volatile, white solid that is used in enriching uranium for nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s and nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission or atomic bomb) or a combination of fission and fusion reactions (thermonuclear weapon), producing a nuclear exp ...
s.
Preparation
Uranium dioxide
Uranium dioxide or uranium(IV) oxide (), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reac ...
is converted with hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
(HF) to uranium tetrafluoride:
:
The resulting is subsequently oxidized with fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
to give the hexafluoride:
:
In samples contaminated with uranium trioxide, uranyl fluoride, an oxyfluoride compound is produced in the HF step:
:
which can be fluorinated to produce the same product, uranium hexafluoride.
:
The fluorination step in both reactions above are highly exothermic.
Properties
Physical properties
Atatmospheric pressure
Atmospheric pressure, also known as air pressure or barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1,013. ...
, sublimes at 56.5 °C.
The solid-state structure was determined by neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of Neutron temperature, thermal or ...
at 77 K and 293 K.J. C. Taylor, P. W. Wilson, J. W. Kelly: „The structures of fluorides. I. Deviations from ideal symmetry in the structure of crystalline UF6: a neutron diffraction analysis", '' Acta Crystallogr.'', 1973, ''B29'', p. 7–12; .
:
Chemical properties
UF6 reacts with water, releasinghydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
. The compound reacts with aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
, forming a surface layer of that resists any further reaction from the compound.
Uranium hexafluoride is a mild oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ''electr ...
. It is a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
as evidenced by its binding to form heptafluorouranate(VI), .
Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
ic uranium(VI) fluorides containing organic cations have been isolated and characterized by X-ray diffraction.
Application in the fuel cycle
 As one of the most volatile compounds of uranium, uranium hexafluoride is relatively convenient to process and is used in both of the main uranium enrichment methods, namely gaseous diffusion and the gas centrifuge method. Since the
As one of the most volatile compounds of uranium, uranium hexafluoride is relatively convenient to process and is used in both of the main uranium enrichment methods, namely gaseous diffusion and the gas centrifuge method. Since the triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
of ; 64 °C(147 °F; 337 K) and 152 kPa (22 psi; 1.5 atm); is close to ambient conditions, phase transitions can be achieved with little thermodynamic work.
Fluorine has only a single naturally occurring stable isotope, so isotopologues of differ in their molecular weight based solely on the uranium isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
present. This difference is the basis for the physical separation of isotopes in enrichment.
All the other uranium fluorides are nonvolatile solids that are coordination polymers.
The conversion factor for the isotopologue of ("hex") to "U mass" is 0.676.
Gaseous diffusion requires about 60 times as much energy as the gas centrifuge process: gaseous diffusion-produced nuclear fuel produces 25 times more energy than is used in the diffusion process, while centrifuge-produced fuel produces 1,500 times more energy than is used in the centrifuge process.
In addition to its use in enrichment, uranium hexafluoride has been used in an advanced reprocessing method ( fluoride volatility), which was developed in the Czech Republic
The Czech Republic, also known as Czechia, and historically known as Bohemia, is a landlocked country in Central Europe. The country is bordered by Austria to the south, Germany to the west, Poland to the northeast, and Slovakia to the south ...
. In this process, spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
is treated with fluorine gas to transform the oxides or elemental metals into a mixture of fluorides. This mixture is then distilled to separate the different classes of material. Some fission products form nonvolatile fluorides which remain as solids and can then either be prepared for storage as nuclear waste or further processed either by solvation
Solvations describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, includi ...
-based methods or electrochemically.
Uranium enrichment produces large quantities of depleted uranium hexafluoride (D or D-) as a waste product. The long-term storage of D- presents environmental, health, and safety risks because of its chemical instability. When is exposed to moist air, it reacts with the water in the air to produce ( uranyl fluoride) and HF (hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
) both of which are highly corrosive and toxic. In 2005, 686,500 tonnes of D- was housed in 57,122 storage cylinders located near Portsmouth, Ohio
Portsmouth is a city in Scioto County, Ohio, United States, and its county seat. Located in southern Ohio south of Chillicothe, Ohio, Chillicothe, it lies on the north bank of the Ohio River, across from Kentucky and just east of the mouth of th ...
; Oak Ridge, Tennessee
Oak Ridge is a city in Anderson County, Tennessee, Anderson and Roane County, Tennessee, Roane counties in the East Tennessee, eastern part of the U.S. state of Tennessee, about west of downtown Knoxville, Tennessee, Knoxville. Oak Ridge's po ...
; and Paducah, Kentucky
Paducah ( ) is a List of cities in Kentucky, home rule-class city in the Upland South, and the county seat of McCracken County, Kentucky, United States. The most populous city in the Jackson Purchase region, it is located in the Southeastern Unit ...
. Storage cylinders must be regularly inspected for signs of corrosion and leaks. The estimated lifetime of the steel cylinders is measured in decades.
Accidents and disposal
There have been several accidents involving uranium hexafluoride in the US, including a cylinder-filling accident and material release at the Sequoyah Fuels Corporation in 1986 where an estimated 29 500 pounds of gaseous escaped. The U.S. government has been converting D to solid uranium oxides for disposal. Such disposal of the entire D stockpile could cost anywhere from $15 million to $450 million.References
Further reading
* '' Gmelins Handbuch der anorganischen Chemie'', System Nr. 55, Uran, Teil A, p. 121–123. * ''Gmelins Handbuch der anorganischen Chemie'', System Nr. 55, Uran, Teil C 8, p. 71–163. * R. DeWitt: ''Uranium hexafluoride: A survey of the physico-chemical properties'', Technical report, GAT-280; Goodyear Atomic Corp., Portsmouth, Ohio; 12. August 1960; . * Ingmar Grenthe, Janusz Drożdżynński, Takeo Fujino, Edgar C. Buck, Thomas E. Albrecht-Schmitt, Stephen F. WolfUranium
, in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Hrsg.): ''The Chemistry of the Actinide and Transactinide Elements'', Springer, Dordrecht 2006; , p. 253–698; (p. 530–531, 557–564). * US-Patent 2535572
26. December 1950. * US-Patent 5723837
3. March 1998.
External links
* Simon Cotton (Uppingham School, Rutland, UK)Uranium Hexafluoride (UF6) – Physical and chemical properties of UF6, and its use in uranium processing – Uranium Hexafluoride and Its Properties
at WebElements
Import of Western depleted uranium hexafluoride (uranium tails) to Russia
ead link 30 June 2017 {{Actinide halides Actinide halides Hexafluorides Nuclear materials Octahedral compounds Uranium(VI) compounds