Unshared Pair on:
[Wikipedia]
[Google]
[Amazon]
 In chemistry, a lone pair refers to a pair of
In chemistry, a lone pair refers to a pair of
''lone (electron) pair''
/ref> and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost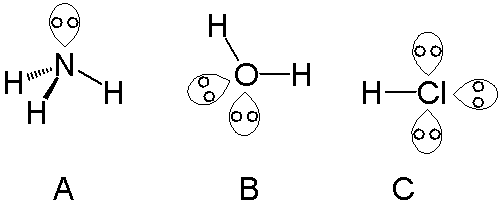 A ''single'' lone pair can be found with atoms in the
A ''single'' lone pair can be found with atoms in the
 The pairs often exhibit a negative polar character with their high charge density and are located closer to the
The pairs often exhibit a negative polar character with their high charge density and are located closer to the

 In elementary chemistry courses, the lone pairs of water are described as "rabbit ears": two equivalent electron pairs of approximately sp3 hybridization, while the HOH bond angle is 104.5°, slightly smaller than the ideal tetrahedral angle of arccos(–1/3) ≈ 109.47°. The smaller bond angle is rationalized by
In elementary chemistry courses, the lone pairs of water are described as "rabbit ears": two equivalent electron pairs of approximately sp3 hybridization, while the HOH bond angle is 104.5°, slightly smaller than the ideal tetrahedral angle of arccos(–1/3) ≈ 109.47°. The smaller bond angle is rationalized by
valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s that are not shared with another atom in a covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
''Gold Book
The International Union of Pure and Applied Chemistry (IUPAC) publishes many books which contain its complete list of definitions. The definitions are divided initially into seven IUPAC Colour Books: Gold, Green, Blue, Purple, Orange, White, and R ...
'' definition''lone (electron) pair''
/ref> and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost
electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (o ...
of atoms. They can be identified by using a Lewis structure
Lewis structuresalso called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs)are diagrams that show the chemical bond, bonding between atoms of a molecule, as well as the lone pairs of elec ...
. Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in ...
. Thus, the number of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s in lone pairs plus the number of electrons in bonds equals the number of valence electrons around an atom.
Lone pair is a concept used in valence shell electron pair repulsion theory
Valence shell electron pair repulsion (VSEPR) theory ( , ) is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theo ...
(VSEPR theory) which explains the shapes of molecules. They are also referred to in the chemistry of Lewis acids and bases
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any s ...
. However, not all non-bonding pairs of electrons are considered by chemists to be lone pairs. Examples are the transition metals where the non-bonding pairs do not influence molecular geometry and are said to be stereochemically inactive. In molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. The MOT explains the paramagnetic nature of O2, whic ...
(fully delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
canonical orbitals or localized in some form), the concept of a lone pair is less distinct, as the correspondence between an orbital and components of a Lewis structure is often not straightforward. Nevertheless, occupied non-bonding orbital
A non-bonding orbital, also known as ''non-bonding molecular orbital'' (NBMO), is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms. Non-bonding orbitals are often designa ...
s (or orbitals of mostly nonbonding character) are frequently identified as lone pairs.
 A ''single'' lone pair can be found with atoms in the
A ''single'' lone pair can be found with atoms in the nitrogen group
, -
! colspan=2 style="text-align:left;" , ↓ Period
, -
! 2
,
, -
! 3
,
, -
! 4
,
, -
! 5
,
, -
! 6
,
, -
! 7
,
, -
, colspan="2",
----
''Legend''
A pnictogen ( or ; from "to choke" and -gen, "generator") is any ...
, such as nitrogen in ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
. ''Two'' lone pairs can be found with atoms in the chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
group, such as oxygen in water. The halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s can carry ''three'' lone pairs, such as in hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
.
In VSEPR theory the electron pairs on the oxygen atom in water form the vertices of a tetrahedron with the lone pairs on two of the four vertices. The H–O–H bond angle
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that deter ...
is 104.5°, less than the 109° predicted for a tetrahedral angle
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertices. The tetrahedron is the simplest of all the ordinary conv ...
, and this can be explained by a repulsive interaction between the lone pairs.
Various computational criteria for the presence of lone pairs have been proposed. While electron density ρ(r) itself generally does not provide useful guidance in this regard, the Laplacian
In mathematics, the Laplace operator or Laplacian is a differential operator given by the divergence of the gradient of a scalar function on Euclidean space. It is usually denoted by the symbols \nabla\cdot\nabla, \nabla^2 (where \nabla is th ...
of the electron density is revealing, and one criterion for the location of the lone pair is where ''L''(r) ''= –''∇2ρ(r) is a local maximum. The minima of the electrostatic potential ''V''(r) is another proposed criterion. Yet another considers the electron localization function
In quantum chemistry, the electron localization function (ELF) is a measure of the likelihood of finding an electron in the neighborhood space of a reference electron located at a given point and with the same Spin (physics), spin. Physically, th ...
(ELF).
Angle changes
 The pairs often exhibit a negative polar character with their high charge density and are located closer to the
The pairs often exhibit a negative polar character with their high charge density and are located closer to the atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the Department_of_Physics_and_Astronomy,_University_of_Manchester , University of Manchester ...
on average compared to the bonding pair of electrons. The presence of a lone pair decreases the bond angle between the bonding pair of electrons, due to their high electric charge, which causes great repulsion between the electrons. They are also involved in the formation of a dative bond
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal io ...
. For example, the creation of the hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the cation , also written as , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved ...
(H3O+) ion occurs when acids are dissolved in water and is due to the oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
atom donating a lone pair to the hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
ion.
This can be seen more clearly when looked at it in two more common molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s. For example, in carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
(CO2), which does not have a lone pair, the oxygen atoms are on opposite sides of the carbon atom (linear molecular geometry
The linear molecular geometry describes the geometry around a central atom bonded to two other atoms (or ''ligands'') placed at a bond angle of 180°. Linear organic molecules, such as acetylene (), are often described by invoking sp orbital ...
), whereas in water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
(H2O) which has two lone pairs, the angle between the hydrogen atoms is 104.5° (bent molecular geometry
In chemistry, molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry, also known as angular or V-shaped. Certain atoms, such as oxygen, will almost always set their two (or more) covalent bonds in non-collin ...
). This is caused by the repulsive force of the oxygen atom's two lone pairs pushing the hydrogen atoms further apart, until the forces of all electrons on the hydrogen atom are in equilibrium
Equilibrium may refer to:
Film and television
* ''Equilibrium'' (film), a 2002 science fiction film
* '' The Story of Three Loves'', also known as ''Equilibrium'', a 1953 romantic anthology film
* "Equilibrium" (''seaQuest 2032'')
* ''Equilibr ...
. This is an illustration of the VSEPR theory
Valence shell electron pair repulsion (VSEPR) theory ( , ) is a conceptual model, model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gill ...
.
Dipole moments
Lone pairs can contribute to a molecule's dipole moment. NH3 has a dipole moment of 1.42 D. As theelectronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
of nitrogen (3.04) is greater than that of hydrogen (2.2) the result is that the N-H bonds are polar with a net negative charge on the nitrogen atom and a smaller net positive charge on the hydrogen atoms. There is also a dipole associated with the lone pair and this reinforces the contribution made by the polar covalent N-H bonds to ammonia's dipole moment. In contrast to NH3, NF3 has a much lower dipole moment of 0.234 D. Fluorine is more electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
than nitrogen and the polarity of the N-F bonds is opposite to that of the N-H bonds in ammonia, so that the dipole due to the lone pair opposes the N-F bond dipoles, resulting in a low molecular dipole moment.
Stereogenic lone pairs
A lone pair can contribute to the existence of chirality in a molecule, when three other groups attached to an atom all differ. The effect is seen in certainamine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s, phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
s, sulfonium
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion (a "cation") featuring three organic Substitution (chemistry), substituents attached to sulfur. These organosulfur compounds have t ...
and oxonium ion
In chemistry, an oxonium ion is any cation containing an oxygen atom that has three chemical bond, bonds and 1+ formal charge. The simplest oxonium ion is the hydronium ion ().
Alkyloxonium
Hydronium is one of a series of oxonium ions with the fo ...
s, sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s, and even carbanion
In organic chemistry, a carbanion is an anion with a lone pair attached to a tervalent carbon atom. This gives the carbon atom a negative charge.
Formally, a carbanion is the conjugate base of a carbon acid:
:
where B stands for the base (chemist ...
s.
The resolution of enantiomers where the stereogenic center is an amine is usually precluded because the energy barrier
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
for nitrogen inversion
In chemistry, pyramidal inversion (also umbrella inversion) is a fluxional process in compounds with a pyramidal molecule, such as ammonia (NH3) "turns inside out". It is a rapid oscillation of the atom and substituents, the molecule or ion pass ...
at the stereo center is low, which allow the two stereoisomers to rapidly interconvert at room temperature. As a result, such chiral amines cannot be resolved, unless the amine's groups are constrained in a cyclic structure (such as in Tröger's base
Tröger's base is a white solid tetracyclic organic compound. Its chemical formula is . Tröger's base and its analogs are soluble in various organic solvents and strong acidic aqueous solutions due to their protonation. It is named after Julius ...
).
Unusual lone pairs
A stereochemically active lone pair is also expected for divalentlead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
and tin
Tin is a chemical element; it has symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the ...
ions due to their formal electronic configuration of n''s''2. In the solid state this results in the distorted metal coordination observed in the tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Pri ...
litharge
Litharge (from Greek , 'stone' + 'silver' ) is one of the natural mineral forms of lead(II) oxide, PbO. Litharge is a secondary mineral which forms from the oxidation of galena ores. It forms as coatings and encrustations with internal tetr ...
structure adopted by both PbO and SnO.
The formation of these heavy metal n''s''2 lone pairs which was previously attributed to intra-atomic hybridization of the metal s and p states has recently been shown to have a strong anion dependence. This dependence on the electronic states of the anion can explain why some divalent lead and tin materials such as PbS and SnTe show no stereochemical evidence of the lone pair and adopt the symmetric rocksalt crystal structure.
In molecular systems the lone pair can also result in a distortion in the coordination of ligands around the metal ion. The lone-pair effect of lead can be observed in supramolecular complexes of lead(II) nitrate
Lead(II) nitrate is an inorganic compound with the chemical formula Pb( NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.
Known since the Middle Ages by the name plumbum ...
, and in 2007 a study linked the lone pair to lead poisoning
Lead poisoning, also known as plumbism and saturnism, is a type of metal poisoning caused by lead in the body. Symptoms may include abdominal pain, constipation, headaches, irritability, memory problems, infertility, numbness and paresthesia, t ...
. Lead ions can replace the native metal ions in several key enzymes, such as zinc cations in the ALAD
Aminolevulinic acid dehydratase (porphobilinogen synthase, or ALA dehydratase, or aminolevulinate dehydratase) is an enzyme () that in humans is encoded by the ''ALAD'' gene. Porphobilinogen synthase (or ALA dehydratase, or aminolevulinate dehydra ...
enzyme, which is also known as porphobilinogen synthase
Aminolevulinic acid dehydratase (porphobilinogen synthase, or ALA dehydratase, or aminolevulinate dehydratase) is an enzyme () that in humans is encoded by the ''ALAD'' gene. Porphobilinogen synthase (or ALA dehydratase, or aminolevulinate dehydra ...
, and is important in the synthesis of heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
, a key component of the oxygen-carrying molecule hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
. This inhibition of heme synthesis appears to be the molecular basis of lead poisoning (also called "saturnism" or "plumbism").
Computational experiments reveal that although the coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
does not change upon substitution in calcium-binding proteins, the introduction of lead distorts the way the ligands organize themselves to accommodate such an emerging lone pair: consequently, these proteins are perturbed. This lone-pair effect becomes dramatic for zinc-binding proteins, such as the above-mentioned porphobilinogen synthase, as the natural substrate cannot bind anymore – in those cases the protein is inhibited.
In Group 14 elements (the carbon group
The carbon group is a group (periodic table), periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
In modern International Union of Pure and Applied Ch ...
), lone pairs can manifest themselves by shortening or lengthening single bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of th ...
(bond order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between t ...
1) lengths, as well as in the effective order of triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, sin ...
s as well. The familiar alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s have a carbon-carbon triple bond (bond order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between t ...
3) and a linear geometry of 180° bond angles (figure A in reference ). However, further down in the group (silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
, germanium
Germanium is a chemical element; it has Symbol (chemistry), symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid or a nonmetal in the carbon group that is chemically ...
, and tin
Tin is a chemical element; it has symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the ...
), formal triple bonds have an effective bond order 2 with one lone pair (figure B) and trans
Trans- is a Latin prefix meaning "across", "beyond", or "on the other side of".
Used alone, trans may refer to:
Sociology
* Trans, a sociological term which may refer to:
** Transgender, people who identify themselves with a gender that di ...
-bent geometries. In lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
, the effective bond order is reduced even further to a single bond, with two lone pairs for each lead atom (figure ''C''). In the organogermanium compound
Organogermanium chemistry is the science of chemical species containing one or more Carbon, C–Germanium, Ge bonds. Germanium shares Carbon group, group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are ...
(''Scheme 1'' in the reference), the effective bond order is also 1, with complexation of the acidic
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
The first category of acids are the ...
isonitrile
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragme ...
(or ''isocyanide'') C-N groups, based on interaction with germanium's empty 4p orbital.

Different descriptions for multiple lone pairs
 In elementary chemistry courses, the lone pairs of water are described as "rabbit ears": two equivalent electron pairs of approximately sp3 hybridization, while the HOH bond angle is 104.5°, slightly smaller than the ideal tetrahedral angle of arccos(–1/3) ≈ 109.47°. The smaller bond angle is rationalized by
In elementary chemistry courses, the lone pairs of water are described as "rabbit ears": two equivalent electron pairs of approximately sp3 hybridization, while the HOH bond angle is 104.5°, slightly smaller than the ideal tetrahedral angle of arccos(–1/3) ≈ 109.47°. The smaller bond angle is rationalized by VSEPR theory
Valence shell electron pair repulsion (VSEPR) theory ( , ) is a conceptual model, model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gill ...
by ascribing a larger space requirement for the two identical lone pairs compared to the two bonding pairs. In more advanced courses, an alternative explanation for this phenomenon considers the greater stability of orbitals with excess s character using the theory of isovalent hybridization, in which bonds and lone pairs can be constructed with sp''x'' hybrids wherein nonintegral values of ''x'' are allowed, so long as the total amount of s and p character is conserved (one s and three p orbitals in the case of second-row p-block elements).
To determine the hybridization of oxygen orbitals used to form the bonding pairs and lone pairs of water in this picture, we use the formula 1 + ''x'' cos θ = 0, which relates bond angle θ with the hybridization index ''x''. According to this formula, the O–H bonds are considered to be constructed from O bonding orbitals of ~sp4.0 hybridization (~80% p character, ~20% s character), which leaves behind O lone pairs orbitals of ~sp2.3 hybridization (~70% p character, ~30% s character). These deviations from idealized sp3 hybridization (75% p character, 25% s character) for tetrahedral geometry are consistent with Bent's rule
In chemistry, Bent's rule describes and explains the relationship between the orbital hybridization and the electronegativities of substituents. The rule was stated by Henry A. Bent as follows:
Valence bond theory gives a good approximation ...
: lone pairs localize more electron density closer to the central atom compared to bonding pairs; hence, the use of orbitals with excess s character to form lone pairs (and, consequently, those with excess p character to form bonding pairs) is energetically favorable.
However, theoreticians often prefer an alternative description of water that separates the lone pairs of water according to symmetry with respect to the molecular plane. In this model, there are two energetically and geometrically distinct lone pairs of water possessing different symmetry: one (σ) in-plane and symmetric with respect to the molecular plane and the other (π) perpendicular and anti-symmetric with respect to the molecular plane. The σ-symmetry lone pair (σ(out)) is formed from a hybrid orbital that mixes 2s and 2p character, while the π-symmetry lone pair (p) is of exclusive 2p orbital parentage. The s character rich O σ(out) lone pair orbital (also notated ''n''O(σ)) is an ~sp0.7 hybrid (~40% p character, 60% s character), while the p lone pair orbital (also notated ''n''O(π)) consists of 100% p character.
Both models are of value and represent the same total electron density, with the orbitals related by a unitary transformation
In mathematics, a unitary transformation is a linear isomorphism that preserves the inner product: the inner product of two vectors before the transformation is equal to their inner product after the transformation.
Formal definition
More precise ...
. In this case, we can construct the two equivalent lone pair hybrid orbitals ''h'' and ''h''properties of water
Water () is a Chemical polarity, polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from Color of water, an inherent hint of blue. It is by far the most studied chemical compou ...
that depend on the ''overall'' electron distribution of the molecule, the use of ''h'' and ''h''anomeric effect
In organic chemistry, the anomeric effect or Edward-Lemieux effect (after J. T. Edward and Raymond Lemieux) is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to the heteroatom in the ring in, e.g., t ...
can be rationalized using equivalent lone pairs, since it is the ''overall'' donation of electron density into the antibonding orbital that matters. An alternative treatment using σ/π separated lone pairs is also valid, but it requires striking a balance between maximizing ''n''O(π)-σ* overlap (maximum at 90° dihedral angle) and ''n''O(σ)-σ* overlap (maximum at 0° dihedral angle), a compromise that leads to the conclusion that a ''gauche'' conformation (60° dihedral angle) is most favorable, the same conclusion that the equivalent lone pairs model rationalizes in a much more straightforward manner. Similarly, the hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s of water form along the directions of the "rabbit ears" lone pairs, as a reflection of the increased availability of electrons in these regions. This view is supported computationally. However, because only the symmetry-adapted canonical orbitals have physically meaningful energies, phenomena that have to do with the energies of ''individual'' orbitals, such as photochemical reactivity or photoelectron spectroscopy
Photoemission spectroscopy (PES), also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in t ...
, are most readily explained using σ and π lone pairs that respect the molecular symmetry
In chemistry, molecular symmetry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explai ...
.
Because of the popularity of VSEPR theory
Valence shell electron pair repulsion (VSEPR) theory ( , ) is a conceptual model, model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gill ...
, the treatment of the water lone pairs as equivalent is prevalent in introductory chemistry courses, and many practicing chemists continue to regard it as a useful model. A similar situation arises when describing the two lone pairs on the carbonyl oxygen atom of a ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
. However, the question of whether it is conceptually useful to derive equivalent orbitals from symmetry-adapted ones, from the standpoint of bonding theory and pedagogy, is still a controversial one, with recent (2014 and 2015) articles opposing and supporting the practice.
See also
*Coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
*HOMO and LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
(highest occupied molecular orbital and lowest unoccupied molecular orbital)
* Inert-pair effect
*Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
*Shared pair
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
References
{{Chemical bonding theory Chemical bonding