Several methods exist for storing
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H
2 upon demand. While large amounts of hydrogen are produced by various industries, it is mostly consumed at the site of production, notably for the synthesis of
ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
. For many years hydrogen has been stored as compressed gas or
cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th International Institute of Refrigeration's (IIR) International Congress of Refrigeration (held in Washington, DC in 1971) endorsed a univers ...
liquid, and transported as such in cylinders, tubes, and cryogenic tanks for use in industry or as propellant in space programs. The overarching challenge is the very low boiling point of H
2: it boils around 20.268
K (−252.882 °C or −423.188 °F). Achieving such low temperatures requires expending significant energy.
Although molecular hydrogen has very high energy density on a mass basis, partly because of its low
molecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
, as a gas at ambient conditions it has very low energy density by volume. If it is to be used as fuel stored on board a vehicle, pure hydrogen gas must be stored in an energy-dense form to provide sufficient driving range. Because hydrogen is the smallest molecule, it easily escapes from containers. Its effective 100-year
global warming potential
Global warming potential (GWP) is a measure of how much heat a greenhouse gas traps in the atmosphere over a specific time period, relative to carbon dioxide (). It is expressed as a multiple of warming caused by the same mass of carbon dioxide ( ...
(GWP100) is estimated to be .
Established technologies

Compressed hydrogen
Compressed hydrogen
Compressed hydrogen (CH2, CGH2 or CGH2) is the gaseous state of the element hydrogen kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar (5,000 psi) and 700 bar (10,000 psi) is used for mobile hydrogen storage in hydrogen vehi ...
is a storage form whereby hydrogen gas is kept under pressures to increase the storage density. Compressed hydrogen in hydrogen tanks at 350 bar (5,000 psi) and 700 bar (10,000 psi) are used for hydrogen tank systems in vehicles, based on type IV carbon-composite technology.
Car manufacturers including Honda and Nissan have been developing this solution.
Liquefied hydrogen
Liquid hydrogen
Liquid hydrogen () is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecule, molecular H2 form.
To exist as a liquid, H2 must be cooled below its critical point (thermodynamics), critical point of 33 Kelvins, ...
tanks for cars, producing for example the
BMW Hydrogen 7. Japan has a liquid hydrogen (LH2) storage site in Kobe port. Hydrogen is liquefied by reducing its temperature to −253 °C, similar to
liquefied natural gas
Liquefied natural gas (LNG) is natural gas (predominantly methane, CH4, with some mixture of ethane, C2H6) that has been cooled to liquid form for ease and safety of non-pressurized storage or transport. It takes up about 1/600th the volume o ...
(LNG) which is stored at −162 °C. A potential efficiency loss of only 12.79% can be achieved, or 4.26 kW⋅h/kg out of 33.3 kW⋅h/kg.
Chemical storage

Chemical storage could offer high storage performance due to the high storage densities. For example, supercritical hydrogen at 30 °C and 500 bar only has a density of 15.0 mol/L while
methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
has a hydrogen density of 49.5 mol H
2/L methanol and saturated
dimethyl ether
Dimethyl ether (DME; also known as methoxymethane) is the organic compound with the formula CH3OCH3,
(sometimes ambiguously simplified to C2H6O as it is an isomer of ethanol). The simplest ether, it is a colorless gas that is a useful precursor ...
at 30 °C and 7 bar has a density of 42.1 mol H
2/L dimethyl ether.
Regeneration of storage material is problematic. A large number of chemical storage systems have been investigated. H
2 release can be induced by
hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
reactions or catalyzed
dehydrogenation reactions. Illustrative storage compounds are hydrocarbons,
boron hydride
Diborane(6), commonly known as diborane, is the chemical compound with the formula . It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attr ...
s,
ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, and
alane etc. A most promising chemical approach is electrochemical hydrogen storage, as the release of hydrogen can be controlled by the applied electricity. Most of the materials listed below can be directly used for electrochemical hydrogen storage.
Nanomaterials
Nanomaterials describe, in principle, chemical substances or materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale).
Nanomaterials research takes a materials science ...
, particularly those produced by
ball mill
A ball mill is a type of grinder filled with grinding balls, used to grind or blend materials for use in mineral dressing processes, paints, pyrotechnics, ceramics, and selective laser sintering. It works on the principle of impact and attri ...
and
severe plastic deformation, offer an alternative that overcomes the two major barriers of bulk materials, rate of sorption and activation.
High-entropy alloy materials such as TiZrCrMnFeNi also show advantages of fast and reversible hydrogen storage at room temperature with good storage capacity for stationary applications.
Enhancement of
sorption
Sorption is a physical and chemical process by which one substance becomes attached to another. Specific cases of sorption are treated in the following articles:
; Absorption: "the incorporation of a substance in one state into another of a d ...
kinetics and storage capacity can be improved through
nanomaterial-based catalyst doping, as shown in the work of the Clean Energy Research Center in the
University of South Florida
The University of South Florida (USF) is a Public university, public research university with its main campus located in Tampa, Florida, Tampa, Florida, United States, and other campuses in St. Petersburg, Florida, St. Petersburg and Sarasota, ...
.
This research group studied LiBH
4 doped with
nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
s and analyzed the weight loss and release temperature of the different species. They observed that an increasing amount of nanocatalyst lowers the release temperature by approximately 20 °C and increases the weight loss of the material by 2-3%. The optimum amount of Ni particles was found to be 3 mol%, for which the temperature was within the limits established (around 100 °C) and the weight loss was notably greater than the undoped species.
The rate of hydrogen sorption improves at the nanoscale due to the short
diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
distance in comparison to bulk materials. They also have favorable
surface-area-to-volume ratio
The surface-area-to-volume ratio or surface-to-volume ratio (denoted as SA:V, SA/V, or sa/vol) is the ratio between surface area and volume of an object or collection of objects.
SA:V is an important concept in science and engineering. It is use ...
.
The release temperature of a material is defined as the temperature at which the
desorption
Desorption is the physical process where Adsorption, adsorbed atoms or molecules are released from a surface into the surrounding vacuum or fluid. This occurs when a molecule gains enough energy to overcome the activation barrier and the binding e ...
process begins. The energy or temperature to induce release affects the cost of any chemical storage strategy. If the hydrogen is bound too weakly, the pressure needed for regeneration is high, thereby cancelling any energy savings. The target for onboard hydrogen fuel systems is roughly <100 °C for release and <700 bar for recharge (20–60 kJ/mol H
2). A modified
van 't Hoff equation
The Van 't Hoff equation relates the change in the equilibrium constant, , of a chemical reaction to the change in temperature, ''T'', given the standard enthalpy change, , for the process. The subscript r means "reaction" and the superscript \om ...
, relates temperature and partial pressure of hydrogen during the desorption process. The modifications to the standard equation are related to size effects at the nanoscale.
Where is the partial pressure of hydrogen, is the
enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
of the sorption process (exothermic), is the change in
entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
, is the ideal
gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment p ...
, T is the temperature in Kelvin, is the
molar volume
In chemistry and related fields, the molar volume, symbol ''V''m, or \tilde V of a substance is the ratio of the volume (''V'') occupied by a substance to the amount of substance (''n''), usually at a given temperature and pressure. It is also eq ...
of the metal, is the radius of the nanoparticle and is the
surface free energy of the particle.
From the above relation we see that the enthalpy and entropy change of desorption processes depend on the radius of the nanoparticle. Moreover, a new term is included that takes into account the specific surface area of the particle and it can be mathematically proven that a decrease in particle radius leads to a decrease in the release temperature for a given partial pressure.
Hydrogenation of CO2
Hydrogenation of CO
2 to methanol has been evaluated for hydrogen storage. Barriers of CO
2 hydrogenation includes purification of captured CO
2, H
2 source from splitting water and energy inputs for hydrogenation. For industrial applications, CO
2 is often converted to methanol. Until now, much progress has been made for CO
2 to C1 molecules. However, CO
2 to high value molecules still face many roadblocks and the future of CO
2 hydrogenation depends on the advancement of catalytic technologies.
Metal hydrides
Metal hydrides
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all co ...
, such as
MgH2,
NaAlH4,
LiAlH4,
LiH,
LaNi5H6,
TiFeH2,
ammonia borane
Ammonia borane (also systematically named ammoniotrihydroborate), also called borazane, is the chemical compound with the formula . The colourless or white solid is the simplest molecular boron-nitrogen-hydride compound. It has attracted attention ...
, and
palladium hydride
Palladium hydride is palladium metal with hydrogen within its crystal lattice. Despite its name, it is not an ionic hydride but rather an alloy of palladium with metallic hydrogen that can be written PdH. At room temperature, palladium hydrides ma ...
represent sources of stored hydrogen. There are three main classes of metal hydrides:
* Inter-metallic Hydrides: exhibit fast kinetics and moderate hydrogen capacities. Such as
LaNi5H6,
TiFeH2.
* Complex Hydrides: capable of higher hydrogen storage capacities but require catalysts. Such as
NaAlH4,
LiBH4.
* Lightweight Hydrides: offer high gravimetric hydrogen storage but require high temperatures for desorption. Such as
MgH2,
CaH2.
Here are the properties of some metal hydrides:
Again the persistent problems are the % weight of H
2 that they carry and the reversibility of the storage process. Some are easy-to-fuel liquids at ambient temperature and pressure, whereas others are solids which could be turned into pellets. These materials have good
energy density
In physics, energy density is the quotient between the amount of energy stored in a given system or contained in a given region of space and the volume of the system or region considered. Often only the ''useful'' or extractable energy is measure ...
, although their
specific energy
Specific energy or massic energy is energy per unit mass. It is also sometimes called gravimetric energy density, which is not to be confused with energy density, which is defined as energy per unit volume. It is used to quantify, for example, st ...
is often worse than the leading
hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
fuels.
An alternative method for lowering dissociation temperatures is doping with activators. This strategy has been used for
aluminium hydride, but the complex synthesis makes the approach unattractive.
Proposed hydrides for use in a
hydrogen economy
The hydrogen economy is an umbrella term for the roles hydrogen can play alongside low-carbon electricity to reduce emissions of greenhouse gases. The aim is to reduce emissions where cheaper and more energy-efficient clean solutions are not ava ...
include simple hydrides of
magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
or
transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s and
complex metal hydrides, typically containing
sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
,
lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
, or
calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
and
aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
or
boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
. Hydrides chosen for storage applications provide low reactivity (high safety) and high hydrogen storage densities. Leading candidates are
lithium hydride
Lithium hydride is an inorganic compound with the formula Li H. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not solub ...
,
sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
,
lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula or . It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthe ...
and
ammonia borane
Ammonia borane (also systematically named ammoniotrihydroborate), also called borazane, is the chemical compound with the formula . The colourless or white solid is the simplest molecular boron-nitrogen-hydride compound. It has attracted attention ...
. A French company McPhy Energy is developing the first industrial product, based on magnesium hydride, already sold to some major clients such as Iwatani and ENEL.
Reversible hydrogen storage is exhibited by
frustrated Lewis pairs.
The phosphino-borane on the left accepts one equivalent of hydrogen at one atmosphere and 25 °C and expels it again by heating to 100 °C. The storage capacity is 0.25 wt%.
Selected advances using metal hydrides
Aluminium
Hydrogen is produced by hydrolysis of aluminium. It was previously believed that, to react with water, aluminium must be stripped of its natural
oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
passivation layer,
or mixing with
gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875,
elemental gallium is a soft, silvery metal at standard temperature and pressure. ...
(which produces aluminium nanoparticles that allow 90% of the aluminium to react). It has since been demonstrated that efficient reaction is possible by increasing the temperature and pressure of the reaction. The byproduct of the reaction to create hydrogen is
aluminium oxide
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several Aluminium oxide (compounds), aluminium oxides, and specifically identified as alum ...
, which can be recycled back into aluminium with the
Hall–Héroult process
The Hall–Héroult process is the major industrial process for smelting aluminium. It involves dissolving aluminium oxide (alumina) (obtained most often from bauxite, aluminium's chief ore, through the Bayer process) in molten cryolite and e ...
, making the reaction theoretically renewable. Although this requires electrolysis, which consumes a large amount of energy, the energy is then stored in the aluminium (and released when the aluminium is reacted with water).
Magnesium
Traditional
MgH2 stores 7.6 wt% hydrogen, but its high desorption temperature (>300 °C) limits applications. Mg-Ti-V nanocomposites can lower the desorption temperature to below 200 °C. Carbon-coordinated
MgH2 exhibits 80% of improvement on cycling stability over 1000 cycles.
LiBH4 +
MgH2 composites stored about 11 wt% of hydrogen, one of the highest capacities reported. And
ammonia borane (H₃NBH₃) releases 12 wt% hydrogen at moderate temperatures (~100–150 °C).
Mg-based hydrogen storage materials include pure Mg, Mg-based alloys, and Mg-based composites. Nonetheless, the inferior hydrogen absorption/desorption kinetics rooting in the overly undue thermodynamic stability of metal hydride make the Mg-based hydrogen storage alloys currently not appropriate for the real applications, and therefore, massive attempts have been dedicated to overcoming these shortages. Some sample preparation methods, such as smelting, powder sintering, diffusion, mechanical alloying, the hydriding combustion synthesis method, surface treatment, and heat treatment, etc., have been broadly employed for altering the dynamic performance and cycle life of Mg-based hydrogen storage alloys. Besides, some intrinsic modification strategies, including alloying, nanostructuring, doping by catalytic additives, and acquiring nanocomposites with other hydrides, etc., have been mainly explored for intrinsically boosting the performance of Mg-based hydrogen storage alloys.
Like aluminium, magnesium also reacts with water to produce hydrogen.
Of the primary hydrogen storage alloys progressed formerly, Mg and Mg-based hydrogen storage materials are believed to provide the remarkable possibility of the practical application, on account of the advantages as following: 1) the resource of Mg is plentiful and economical. Mg element exists abundantly and accounts for ≈2.35% of the earth's crust with the rank of the eighth; 2) low density of merely 1.74 g cm-3; 3) superior hydrogen storage capacity. The theoretical hydrogen storage amounts of the pure Mg is 7.6 wt % (weight percent), and the Mg2Ni is 3.6 wt%, respectively.
Alanates-based systems
Lithium alanate (LiAlH
4) was synthesized for the first time in 1947 by dissolution of lithium hydride in an ether solution of aluminium chloride. LiAlH
4 has a theoretical gravimetric capacity of 10.5 wt %H
2 and dehydrogenates in the following three steps: 3LiAlH
4 ↔ Li
3AlH
6 + 3H
2 + 2Al (423–448 K; 5.3 wt %H
2; ∆H = −10 kJ·mol−1 H
2); Li
3AlH
6 ↔ 3LiH + Al + 1.5H
2 (453–493 K; 2.6 wt %H
2; ∆H = 25 kJ·mol−1 H
2); 3LiH + 3Al ↔ 3LiAl + 3/2H
2 (>673 K; 2.6 wt %H
2; ∆H = 140 kJ·mol−1 H
2).
[ Text was copied from this source, which is available under ]
Creative Commons Attribution 4.0 International License
. The first two steps lead to a total amount of hydrogen released equal to 7.9 wt %, which could be attractive for practical applications, but the working temperatures and the desorption kinetics are still far from the practical targets. Several strategies have been applied in the last few years to overcome these limits, such as ball-milling and catalysts additions.
Potassium Alanate (KAlH
4) was first prepared by Ashby et al. by one-step synthesis in toluene, tetrahydrofuran, and diglyme. Concerning the hydrogen absorption and desorption properties, this alanate was only scarcely studied. Morioka et al., by temperature programmed desorption (TPD) analyses, proposed the following dehydrogenation mechanism: 3KAlH
4 →K
3AlH
6 + 2Al + 3H
2 (573 K, ∆H = 55 kJ·mol−1 H
2; 2.9 wt %H
2), K
3AlH
6 → 3KH + Al + 3/2H
2 (613 K, ∆H = 70 kJ·mol−1 H
2; 1.4 wt %H
2), 3KH → 3K + 3/2H
2 (703 K, 1.4 wt %H
2). These reactions were demonstrated reversible without catalysts addition at relatively low hydrogen pressure and temperatures. The addition of TiCl3 was found to decrease the working temperature of the first dehydrogenation step of 50 K, but no variations were recorded for the last two reaction steps.
Organic hydrogen carriers
Unsaturated organic compounds can store huge amounts of hydrogen. These ''Liquid Organic Hydrogen Carriers'' (LOHC) are hydrogenated for storage and dehydrogenated again when the energy/hydrogen is needed. Using LOHCs, relatively high gravimetric storage densities can be reached (about 6 wt-%) and the overall
energy efficiency is higher than for other chemical storage options such as
producing methane from the hydrogen. Both hydrogenation and dehydrogenation of LOHCs requires catalysts.
It was demonstrated that replacing hydrocarbons by hetero-atoms, like N, O etc. improves reversible de/hydrogenation properties.
Cycloalkanes
Cycloalkanes have relatively high hydrogen capacity (6-8 wt %).
(or N-Heterocycles) are also appropriate for this task. but many others do exist
Dibenzyltoluene which is already used as a heat transfer fluid in industry, was identified as potential LOHC. With a wide liquid range between -39 °C (melting point) and 390 °C (boiling point) and a hydrogen storage density of 6.2 wt% dibenzyltoluene is ideally suited as LOHC material.
Formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
has been suggested as a promising hydrogen storage material with a 4.4wt% hydrogen capacity.
Cycloalkanes reported as LOHC include cyclohexane, methyl-cyclohexane and decalin. The dehydrogenation of cycloalkanes is highly endothermic (63-69 kJ/mol H
2), which means this process requires high temperature.
Dehydrogenation of decalin is the most thermodynamically favored among the three cycloalkanes, and methyl-cyclohexane is second because of the presence of the methyl group. The dehydrogenation of cycloalkanes is a mature area. Nickel-, molybdenum-, andcat platinum-based catalysts are established.
Coking
Coking is the process of heating coal in the absence of oxygen to a temperature above to drive off the volatile components of the raw coal, leaving behind a hard, strong, porous material with a high carbon content called coke. Coke is predomina ...
remains a challenge.
N-Heterocycles
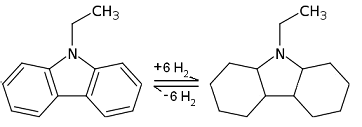
The temperature required for hydrogenation and dehydrogenation drops significantly for heterocycles vs simple carbocycles. Among all the N-heterocycles, the saturated-unsaturated pair of dodecahydro-N-ethylcarbazole (12H-NEC) and NEC has been considered as a promising candidate for hydrogen storage with a fairly large hydrogen content (5.8wt%). The figure on the top right shows dehydrogenation and hydrogenation of the 12H-NEC and NEC pair. The standard catalyst for NEC to 12H-NEC is Ru and Rh based. The selectivity of hydrogenation can reach 97% at 7 MPa and 130 °C-150 °C.
Although N-Heterocyles can optimize the unfavorable thermodynamic properties of cycloalkanes, a lot of issues remain unsolved, such as high cost, high toxicity and kinetic barriers etc.
The imidazolium ionic liquids such alkyl(aryl)-3-methylimidazolium N-bis(trifluoromethanesulfonyl)imidate salts can reversibly add 6–12 hydrogen atoms in the presence of classical Pd/C or Ir0 nanoparticle catalysts and can be used as alternative materials for on-board hydrogen-storage devices. These salts can hold up to 30 g L
−1 of hydrogen at atmospheric pressure.
Formic acid
Formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
is a highly effective hydrogen storage material, although its H
2density is low. Carbon monoxide free hydrogen has been generated in a very wide pressure range (1–600 bar). A homogeneous catalytic system based on water-soluble ruthenium catalysts selectively decompose HCOOH into H
2 and CO
2 in aqueous solution. This catalytic system overcomes the limitations of other catalysts (e.g. poor stability, limited catalytic lifetimes, formation of CO) for the decomposition of formic acid making it a viable hydrogen storage material. And the co-product of this decomposition, carbon dioxide, can be used as hydrogen vector by hydrogenating it back to formic acid in a second step. The catalytic hydrogenation of CO
2 has long been studied and efficient procedures have been developed. Formic acid contains 53 g L
−1 hydrogen at room temperature and atmospheric pressure. By weight, pure formic acid stores 4.3 wt% hydrogen. Pure formic acid is a liquid with a flash point 69 °C (cf. gasoline −40 °C, ethanol 13 °C). 85% formic acid is not flammable.
Ammonia and related compounds
Ammonia
Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
(NH
3) releases H
2 in an appropriate catalytic reformer. Ammonia provides high hydrogen storage densities as a liquid with mild pressurization and cryogenic constraints: It can also be stored as a liquid at room temperature and pressure when mixed with water. Ammonia is the second most commonly produced chemical in the world and a large infrastructure for making, transporting, and distributing ammonia exists. Ammonia can be reformed to produce hydrogen with no harmful waste, or can mix with existing fuels and under the right conditions burn efficiently. Since there is no carbon in ammonia, no carbon by-products are produced; thereby making this possibility a "carbon neutral" option for the future. Pure ammonia burns poorly at the atmospheric pressures found in natural gas fired water heaters and stoves. Under compression in an automobile engine it is a suitable fuel for slightly modified gasoline engines. Ammonia is a suitable alternative fuel because it has 18.6 MJ/kg energy density at NTP and carbon-free combustion byproducts.
Ammonia has several challenges to widespread adaption as a hydrogen storage material. Ammonia is a toxic gas with a potent odor at standard temperature and pressure. Additionally, advances in the efficiency and scalability of ammonia decomposition are needed for commercial viability, as fuel cell membranes are highly sensitive to residual ammonia and current decomposition techniques have low yield rates. A variety of transition metals can be used to catalyze the ammonia decomposition reaction, the most effective being
ruthenium
Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chem ...
. This catalysis works through
chemisorption, where the adsorption energy of N
2 is less than the reaction energy of dissociation. Hydrogen purification can be achieved in several ways. Hydrogen can be separated from unreacted ammonia using a permeable, hydrogen-selective membrane. It can also be purified through the adsorption of ammonia, which can be selectively trapped due to its polarity.
In September 2005 chemists from the
Technical University of Denmark
The Technical University of Denmark (), often simply referred to as DTU, is a polytechnic university and school of engineering. It was founded in 1829 at the initiative of Hans Christian Ørsted as Denmark's first polytechnic, and it is today ran ...
announced a method of storing hydrogen in the form of
ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
saturated into a salt tablet. They claim it will be an inexpensive and safe storage method.
=Positive attributes of Ammonia
=
* High theoretical energy density
* Wide spread availability
* Large scale commercial production
* Benign decomposition pathway to H
2 and N
2
=Negative attributes of Ammonia
=
* Toxicity
* Corrosive
* High decomposition temperature leading to efficiency loss
Hydrazine
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
breaks down in the cell to form
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
/
Silicon hydrides and germanium hydrides are also candidates of hydrogen storage materials, as they can subject to energetically favored reaction to form covalently bonded dimers with loss of a hydrogen molecule.
Amine boranes
Prior to 1980, several compounds were investigated for hydrogen storage including complex borohydrides, or aluminohydrides, and ammonium salts. These hydrides have an upper theoretical hydrogen yield limited to about 8.5% by weight. Amongst the compounds that contain only B, N, and H (both positive and negative ions), representative examples include: amine boranes, boron hydride ammoniates, hydrazine-borane complexes, and ammonium octahydrotriborates or tetrahydroborates. Of these, amine boranes (and especially
ammonia borane
Ammonia borane (also systematically named ammoniotrihydroborate), also called borazane, is the chemical compound with the formula . The colourless or white solid is the simplest molecular boron-nitrogen-hydride compound. It has attracted attention ...
) have been extensively investigated as hydrogen carriers. During the 1970s and 1980s, the U.S. Army and Navy funded efforts aimed at developing hydrogen/deuterium gas-generating compounds for use in the HF/DF and HCl chemical
laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
s, and gas dynamic lasers. Earlier hydrogen gas-generating formulations used amine boranes and their derivatives. Ignition of the amine borane(s) forms
boron nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula B N. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexago ...
(BN) and hydrogen gas. In addition to ammonia borane
(H
3BNH
3), other gas-generators include diborane diammoniate, H
2B(NH
3)
2BH
4.
Physical storage
In this case hydrogen remains in physical forms, i.e., as gas, supercritical fluid, adsorbate, or molecular inclusions. Theoretical limitations and experimental results are considered concerning the volumetric and gravimetric capacity of glass microvessels, microporous, and nanoporous media, as well as safety and refilling-time demands. Because hydrogen is the smallest molecule, it easily escapes from containers and during transfer from container to container. While it does not directly contribute to
radiative forcing
Radiative forcing (or climate forcing) is a concept used to quantify a change to the balance of energy flowing through a planetary atmosphere. Various factors contribute to this change in energy balance, such as concentrations of greenhouse gases ...
, hydrogen is estimated to have an effective 100-year global warming potential of due to its impact on processes such as
atmospheric methane
Atmospheric methane is the methane present in Earth's atmosphere. The concentration of atmospheric methane is increasing due to methane emissions, and is causing climate change. Methane is one of the most potent greenhouse gases. Methane's radiati ...
oxidation and
tropospheric ozone production.
[Bjørnæs, Christian]
"Global warming potential of hydrogen estimated"
Centre for International Climate and Environmental Research
The CICERO Center for International Climate Research (abbreviated CICERO; ) is an interdisciplinary research centre for climate research and environmental science/environmental studies in Oslo. CICERO was established by the Government of Norway i ...
, June 7, 2023. Retrieved June 15, 2023.
Zeolites
Zeolite
Zeolites are a group of several microporous, crystalline aluminosilicate minerals commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a meta ...
s are microporous and highly crystalline
aluminosilicate
Aluminosilicate refers to materials containing anionic Si-O-Al linkages. Commonly, the associate cations are sodium (Na+), potassium (K+) and protons (H+). Such materials occur as minerals, coal combustion products and as synthetic materials, of ...
materials. As they exhibit cage and tunnel structures, they offer the potential for the encapsulation of
non-polar
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
gases such as H
2. In this system, hydrogen is
physisorbed on the surface of the zeolite pores through a mechanism of adsorption that involves hydrogen being forced into the pores under pressure and low temperature.
Therefore, similar to other porous materials, its hydrogen storage capacity depends on the
BET surface area, pore volume, the interaction of molecular hydrogen with the internal surfaces of the micropores, and working conditions such as pressure and temperature.
Channel diameter is also one of the parameters determining this capacity, especially at high pressure. In this case, an effective material should exhibit a large pore volume and a channel diameter close to the
kinetic diameter
Kinetic diameter is a measure applied to atoms and molecules that expresses the likelihood that a molecule in a gas will collide with another molecule. It is an indication of the size of the molecule as a target. The kinetic diameter is not the s ...
of the hydrogen molecule (d
H=2.89 Å).
Table below shows the hydrogen uptake of several zeolites at liquid nitrogen temperature (77K):
Porous or layered carbon
Activated carbons are highly porous amorphous carbon materials with high apparent surface area. Hydrogen
physisorption
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely wikt:perturb, perturbed upon adsorption.
Overview
The fundamental interacting force of physisorption is Van der Waals ...
can be increased in these materials by increasing the apparent surface area and optimizing pore diameter to around 7 Å. These materials are of particular interest due to the fact that they can be made from waste materials, such as cigarette butts which have shown great potential as precursor materials for high-capacity hydrogen storage materials.
Graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
can store hydrogen efficiently. The H
2 adds to the double bonds giving
graphane. The hydrogen is released upon heating to 450 °C.
Hydrogen carriers based on nanostructured carbon (such as carbon
buckyballs and
nanotubes) have been proposed. However, hydrogen content amounts up to ≈3.0-7.0 wt% at 77K which is far from the value set by US Department of Energy (6 wt% at nearly ambient conditions).
To realize carbon materials as effective hydrogen storage technologies, carbon nanotubes (CNTs) have been doped with
MgH2.
The metal hydride has proven to have a theoretical storage capacity (7.6 wt%) that fulfills the
United States Department of Energy
The United States Department of Energy (DOE) is an executive department of the U.S. federal government that oversees U.S. national energy policy and energy production, the research and development of nuclear power, the military's nuclear w ...
requirement of 6 wt%, but has limited practical applications due to its high release temperature. The proposed mechanism involves the creation of fast diffusion channels by
CNTs within the MgH
2 lattice.
Fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
substances are other carbonaceous nanomaterials that have been tested for hydrogen storage in this center. Fullerene molecules are composed of a C
60 close-caged structure, that allows for hydrogenation of the double bonded carbons leading to a theoretical C
60H
60 isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
with a hydrogen content of 7.7 wt%. However, the release temperature in these systems is high (600 °C).
Metal–organic frameworks
Metal–organic frameworks represent another class of synthetic porous materials that store hydrogen and energy at the molecular level. MOFs are highly crystalline inorganic-organic hybrid structures that contain metal clusters or ions (secondary building units) as nodes and organic ligands as linkers. When guest molecules (solvent) occupying the pores are removed during solvent exchange and heating under vacuum, porous structure of MOFs can be achieved without destabilizing the frame and hydrogen molecules will be adsorbed onto the surface of the pores by physisorption. Compared to traditional zeolites and porous carbon materials, MOFs have very high number of pores and surface area which allow higher hydrogen uptake in a given volume.
Factors influencing hydrogen storage ability
Temperature, pressure and composition of MOFs can influence their hydrogen storage ability. The adsorption capacity of MOFs is lower at higher temperature and higher at lower temperatures. With the rising of temperature, physisorption decreases and chemisorption increases.
For MOF-519 and MOF-520, the isosteric heat of adsorption decreased with pressure increase. For MOF-5, both gravimetric and volumetric hydrogen uptake increased with increase in pressure.
The total capacity may not be consistent with the usable capacity under pressure swing conditions. For instance, MOF-5 and IRMOF-20, which have the highest total volumetric capacity, show the least usable volumetric capacity. Absorption capacity can be increased by modification of structure. For example, the hydrogen uptake of PCN-68 is higher than PCN-61. Porous aromatic frameworks (PAF-1), which is known as a high surface area material, can achieve a higher surface area by doping.
Modification of MOFs
There are many different ways to modify MOFs, such as MOF catalysts, MOF hybrids, MOF with metal centers and doping. MOF catalysts have high surface area, porosity and hydrogen storage capacity. However, the active metal centers are low. MOF hybrids have enhanced surface area, porosity, loading capacity and hydrogen storage capacity. Nevertheless, they are not stable and lack active centers. Doping in MOFs can increase hydrogen storage capacity, but there might be steric effect and inert metals have inadequate stability. There might be formation of interconnected pores and low corrosion resistance in MOFs with metal centers, while they might have good binding energy and enhanced stability. These advantages and disadvantages for different kinds of modified MOFs show that MOF hybrids are more promising because of the good controllability in selection of materials for high surface area, porosity and stability.
In 2006, chemists achieved hydrogen storage concentrations of up to 7.5 wt% in MOF-74 at a low temperature of 77
K. MOF NOTT-112 exhibit 10 wt% at 77 bar (1,117 psi) and 77 K with . Most articles about hydrogen storage in MOFs report hydrogen subitptake capacity at a temperature of 77K and a pressure of 1 bar because these conditions are commonly available and the binding energy between hydrogen and the MOF at this temperature is large compared to the thermal vibration energy. Varying several factors such as surface area, pore size, catenation, ligand structure, and sample purity can result in different amounts of hydrogen uptake in MOFs.
NU-1501-Al, an ultraporous metal–organic framework (MOF) has a hydrogen delivery capacity of 14.0% w/w, 46.2 g/litre.
Cryo-compressed
Cryo-compressed storage of hydrogen is the only technology that meets 2015 DOE targets for volumetric and gravimetric efficiency (see "CcH2" on slide 6 in
[R. K. Ahluwalia, T. Q. Hua, J. K. Peng and R. Kuma]
System Level Analysis of Hydrogen Storage Options
. 2010 DOE Hydrogen Program Review, Washington, DC, June 8–11, 2010).
Furthermore, another study has shown that cryo-compression exhibits interesting cost advantages: ownership cost (price per mile) and storage system cost (price per vehicle) are actually the lowest when compared to any other technology (see third row in slide 13 of).
Like liquid storage, cryo-compressed uses cold hydrogen (20.3 K and slightly above) in order to reach a high energy density. However, the main difference is that, when the hydrogen would warm-up due to heat transfer with the environment ("boil off"), the tank is allowed to go to pressures much higher (up to 350 bars versus a couple of bars for liquid storage). As a consequence, it takes more time before the hydrogen has to vent, and in most driving situations, enough hydrogen is used by the car to keep the pressure well below the venting limit.
Consequently, it has been demonstrated that a high driving range could be achieved with a cryo-compressed tank : more than were driven with a full tank mounted on a hydrogen-fueled engine of
Toyota Prius
The is a Compact car, compact/small family car, small family liftback (supermini/subcompact sedan (car), sedan until 2003) produced by Toyota. The Prius has a Hybrid vehicle drivetrain, hybrid drivetrain, combined with an internal combustion ...
.
As of 2010, the BMW Group has started a thorough component and system level validation of cryo-compressed vehicle storage on its way to a commercial product.
Cryo-supercritical
Clathrate hydrates
H2 caged in a
clathrate hydrate
Clathrate hydrates, or gas hydrates, clathrates, or hydrates, are crystalline water-based solids physically resembling ice, in which small non-polar molecules (typically gases) or polar molecules with large hydrophobic moieties are trapped ins ...
are stable at very high pressures. Some solid H
2-containing hydrates form at ambient temperature and tens of
bars in the presence of
THF. These clathrates have a theoretical maximum hydrogen densities of around 5 wt% and 40 kg/m
3.
Glass capillary arrays
Glass capillary arrays show potential for the safe infusion, storage and controlled release of hydrogen in mobile applications. The C.En technology has achieved the
United States Department of Energy
The United States Department of Energy (DOE) is an executive department of the U.S. federal government that oversees U.S. national energy policy and energy production, the research and development of nuclear power, the military's nuclear w ...
(DOE) 2010 targets for on-board hydrogen storage systems.
DOE 2015 targets can be achieved using flexible glass capillaries and cryo-compressed method of hydrogen storage.
Glass microspheres
Hollow
glass microspheres (HGM) can be utilized for controlled storage and release of hydrogen. HGMs with a diameter of 1 to 100 μm, a density of 1.0 to 2.0 gm/cc and a porous wall with openings of 10 to 1000
angstroms
The angstrom (; ) is a unit of length equal to m; that is, one ten-billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874) ...
are considered for hydrogen storage. The advantages of HGMs for hydrogen storage are that they are nontoxic, light, cheap, recyclable, reversible, easily handled at atmospheric conditions, capable of being stored in a tank, and the hydrogen within is non-explosive.
Each of these HGMs is capable of containing hydrogen up to 150 MPa without the heaviness and bulk of a large pressurized tank. All of these qualities are favorable in vehicular applications. Beyond these advantages, HGMs are seen as a possible hydrogen solution due to hydrogen
diffusivity
Diffusivity is a rate of diffusion, a measure of the rate at which particles or heat or fluids can spread.
It is measured differently for different mediums.
Diffusivity may refer to:
*Thermal diffusivity, diffusivity of heat
*Diffusivity of mass: ...
having a large temperature dependence. At room temperature, the diffusivity is very low, and the hydrogen is trapped in the HGM. The disadvantage of HGMs is that to fill and
outgas hydrogen effectively the temperature must be at least 300 °C which significantly increases the operational cost of HGM in hydrogen storage. The high temperature can be partly attributed to glass being an
insulator and having a low
thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
; this hinders hydrogen
diffusivity
Diffusivity is a rate of diffusion, a measure of the rate at which particles or heat or fluids can spread.
It is measured differently for different mediums.
Diffusivity may refer to:
*Thermal diffusivity, diffusivity of heat
*Diffusivity of mass: ...
, and subsequently a higher temperature is required to achieve the desired storage capacity.
To make this technology more economically viable for commercial use, research is being done to increase the
efficiency
Efficiency is the often measurable ability to avoid making mistakes or wasting materials, energy, efforts, money, and time while performing a task. In a more general sense, it is the ability to do things well, successfully, and without waste.
...
of hydrogen
diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
through the HGMs. One study done by Dalai et al. sought to increase the
thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
of the HGM through
doping the glass with
cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
. In doing so they increased the
thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
from 0.0072 to 0.198 W/m-K at 10 wt% Co. Increases in hydrogen
adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
though were only seen up to 2 wt% Co (0.103 W/m-K) as the
metal oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation state o ...
began to cover pores in the glass shell. This study concluded with a hydrogen storage capacity of 3.31 wt% with 2 wt% Co at 200 °C and 10 bar.
A study done by Rapp and Shelby sought to increase the hydrogen release rate through photo-induced outgassing in doped HGMs in comparison to conventional heating methods. The glass was doped with
optically active
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
metals to interact with the high-intensity
infrared light
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those o ...
. The study found that 0.5 wt% Fe
3O
4 doped 7070
borosilicate glass
Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. Borosilicate glasses are known for having very low coefficients of thermal expansion (≈3 × 10−6 K−1 at 20 °C), ma ...
had hydrogen release increase proportionally to the infrared lamp intensity. In addition to the improvements to diffusivity by infrared alone, reactions between the hydrogen and iron-doped glass increased the Fe
2+/Fe
3+ ratio which increased infrared absorption therefore further increasing the hydrogen yield.
As of 2020, the progress made in studying HGMs has increased its efficiency but it still falls short of Department of Energy targets for this technology. The operation temperatures for both hydrogen adsorption and release are the largest barrier to
commercialization
Commercialisation or commercialization is the process of introducing a new product or production method into commerce—making it available on the market. The term often connotes especially entry into the mass market (as opposed to entry into e ...
.
Stationary hydrogen storage
Unlike mobile applications, hydrogen density is not a huge problem for stationary applications. As for mobile applications, stationary applications can use established technology:
*
Compressed hydrogen
Compressed hydrogen (CH2, CGH2 or CGH2) is the gaseous state of the element hydrogen kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar (5,000 psi) and 700 bar (10,000 psi) is used for mobile hydrogen storage in hydrogen vehi ...
(CGH
2) in a
hydrogen tank
A hydrogen infrastructure is the infrastructure of points of hydrogen production, truck and pipeline transport, and hydrogen stations for the distribution and sale of hydrogen fuel, and thus a crucial prerequisite before a successful commerciali ...
*
Liquid hydrogen
Liquid hydrogen () is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecule, molecular H2 form.
To exist as a liquid, H2 must be cooled below its critical point (thermodynamics), critical point of 33 Kelvins, ...
in a (LH
2)
cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th International Institute of Refrigeration's (IIR) International Congress of Refrigeration (held in Washington, DC in 1971) endorsed a univers ...
hydrogen tank
*
Slush hydrogen Slush hydrogen is a combination of liquid hydrogen and solid hydrogen at the triple point with a lower temperature and a higher density than liquid hydrogen. It is commonly formed by repeating a freeze-thaw process. This is most easily done by brin ...
in a cryogenic hydrogen tank
Underground hydrogen storage
Underground hydrogen storage is the practice of hydrogen storage in
cave
Caves or caverns are natural voids under the Earth's Planetary surface, surface. Caves often form by the weathering of rock and often extend deep underground. Exogene caves are smaller openings that extend a relatively short distance undergrou ...
rns,
salt dome
A salt dome is a type of structural dome formed when salt (or other evaporite minerals) intrudes into overlying rocks in a process known as diapirism. Salt domes can have unique surface and subsurface structures, and they can be discovered us ...
s and depleted oil and gas fields. Large quantities of gaseous hydrogen have been stored in caverns by
ICI for many years without any difficulties. The storage of large quantities of liquid hydrogen underground can function as
grid energy storage
Grid energy storage, also known as large-scale energy storage, are technologies connected to the electrical power grid that store energy for later use. These systems help balance supply and demand by storing excess electricity from variabl ...
. The round-trip efficiency is approximately 40% (vs. 75–80% for
pumped-hydro (PHES)), and the cost is slightly higher than pumped hydro, if only a limited number of hours of storage is required. Another study referenced by a European staff working paper found that for large scale storage, the cheapest option is hydrogen at €140/MWh for 2,000 hours of storage using an electrolyser, salt cavern storage and combined-cycle power plant.
The European project Hyunder indicated in 2013 that for the storage of wind and solar energy an additional 85 caverns are required as it cannot be covered by
PHES and
CAES systems. A German case study on storage of hydrogen in salt caverns found that if the German power surplus (7% of total variable renewable generation by 2025 and 20% by 2050) would be converted to hydrogen and stored underground, these quantities would require some 15 caverns of 500,000 cubic metres each by 2025 and some 60 caverns by 2050 – corresponding to approximately one third of the number of gas caverns currently operated in Germany. In the US, Sandia Labs are conducting research into the storage of hydrogen in depleted oil and gas fields, which could easily absorb large amounts of renewably produced hydrogen as there are some 2.7 million depleted wells in existence.
Underground hydrogen storage is the practice of hydrogen storage in
cave
Caves or caverns are natural voids under the Earth's Planetary surface, surface. Caves often form by the weathering of rock and often extend deep underground. Exogene caves are smaller openings that extend a relatively short distance undergrou ...
rns,
salt dome
A salt dome is a type of structural dome formed when salt (or other evaporite minerals) intrudes into overlying rocks in a process known as diapirism. Salt domes can have unique surface and subsurface structures, and they can be discovered us ...
s and depleted
oil
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) and lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturate ...
/
gas fields. Large quantities of gaseous
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
have been stored in caverns for many years. The storage of large quantities of hydrogen underground in solution-mined
salt dome
A salt dome is a type of structural dome formed when salt (or other evaporite minerals) intrudes into overlying rocks in a process known as diapirism. Salt domes can have unique surface and subsurface structures, and they can be discovered us ...
s,
aquifer
An aquifer is an underground layer of water-bearing material, consisting of permeability (Earth sciences), permeable or fractured rock, or of unconsolidated materials (gravel, sand, or silt). Aquifers vary greatly in their characteristics. The s ...
s, excavated rock caverns, or
mines can function as
grid energy storage
Grid energy storage, also known as large-scale energy storage, are technologies connected to the electrical power grid that store energy for later use. These systems help balance supply and demand by storing excess electricity from variabl ...
, essential for the
hydrogen economy
The hydrogen economy is an umbrella term for the roles hydrogen can play alongside low-carbon electricity to reduce emissions of greenhouse gases. The aim is to reduce emissions where cheaper and more energy-efficient clean solutions are not ava ...
. By using a
turboexpander
A turboexpander, also referred to as a turbo-expander or an expansion turbine, is a centrifugal or axial-flow turbine, through which a high-pressure gas is expanded to produce work that is often used to drive a compressor or generator.
Because ...
the electricity needs for compressed storage on 200 bar amounts to 2.1% of the energy content.
Salt caverns
The Chevron Phillips Clemens Terminal in Texas has stored hydrogen since the 1980s in a solution-mined salt cavern. The cavern roof is about underground. The cavern is a cylinder with a diameter of , a height of , and a usable hydrogen capacity of , or .
Salt caverns are artificially created by injecting water from the surface into a well in the rock salt, where rock salt is a polycrystalline material made of NaCl, halite. Locations such as salt domes or bedded salt are usually picked for salt caverns' creation. Salt caverns can reach a maximum depth of 2000 m and a maximum volume capacity of 1,000,000 m3. The frequency of injection and withdrawal cycles ranges between 10 and 12 cycles per year. The leak rate is around 1%.
Due to the physiochemical properties of the rock salt, salt caverns exhibit multiple advantages. Key characteristics are low water content, low porosity and permeability, and its chemical inertia towards hydrogen.
Permeability is a key parameter in underground hydrogen storage, which affects its ability to seal. Though studies have found dilatancy and extensional fracture can cause significant permeability increase, rock salt crystal's recrystallization, which is a grain boundaries healing process, may contribute to its mechanical stiffness and permeability recovery. Its plastic properties prevent the formation and spread of fractures and protect it from losing its tightness, which is particularly important for hydrogen storage.
Some of the disadvantages of salt caverns include lower storage capacity, large amount of water needed, and the effect of corrosion. Cushion gas is needed to avoid creep due to pressure drop when withdrawing gas from the reservoir. Though the need for cushion gas is relatively small, around 20%, the operational cost can still add up when working with a larger storage capacity. Cost is another big concern, where the cost of construction and operation are still high.
Though people have experience with storing natural gas, storing hydrogen is a lot more complex. Factors such as hydrogen diffusivity in solids cause restrictions in salt cavern storage. Microbial activity is under extensive research worldwide because of its impact on hydrogen loss. As a result of methanogenic bacteria's bacterial metabolism, carbon dioxide and hydrogen are consumed and methane is produced, which leads to the loss of hydrogen stored in the salt caverns.
Development
*
Sandia National Laboratories
Sandia National Laboratories (SNL), also known as Sandia, is one of three research and development laboratories of the United States Department of Energy's National Nuclear Security Administration (NNSA). Headquartered in Kirtland Air Force B ...
released in 2011 a life-cycle cost analysis framework for geologic storage of hydrogen.
* The European project Hyunder indicated in 2013 that for the storage of wind and solar energy an additional 85 caverns are required as it cannot be covered by
pumped-storage hydroelectricity
Pumped-storage hydroelectricity (PSH), or pumped hydroelectric energy storage (PHES), is a type of hydroelectric energy storage used by electric power systems for load balancing (electrical power), load balancing.
A PSH system stores energy i ...
and
compressed air energy storage
Compressed-air-energy storage (CAES) is a way to store energy for later use using compressed air. At a utility scale, energy generated during periods of low demand can be released during peak load periods.
The first utility-scale CAES project ...
systems.
*
ETI released in 2015 a report ''The role of hydrogen storage in a clean responsive power system'' noting that the UK has sufficient salt bed resources to provide tens of GWe.
*
RAG Austria AG finished a hydrogen storage project in a depleted oil and gas field in Austria in 2017, and is conducting its second project "Underground Sun Conversion".
A cavern sized 800 m tall and 50 m diameter can hold hydrogen equivalent to 150 GWh.
Power to gas
Power to gas is a technology which converts
electrical
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
power to a gas
fuel
A fuel is any material that can be made to react with other substances so that it releases energy as thermal energy or to be used for work (physics), work. The concept was originally applied solely to those materials capable of releasing chem ...
. There are two methods: the first is to use the electricity for
water splitting
Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:
Efficient and economical water splitting would be a technological breakthrough that could underpin a hydrogen economy. A version of water splitti ...
and inject the resulting hydrogen into the natural gas grid; the second, less efficient method is used to convert
carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and hydrogen to
methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, (see
natural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
) using
electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
and the
Sabatier reaction
The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures (optimally 300–400 °C) and pressures (perhaps ) in the presence of a nickel catalyst. It was di ...
. A third option is to combine the hydrogen via electrolysis with a source of carbon (either carbon dioxide or carbon monoxide from
biogas
Biogas is a gaseous renewable energy source produced from raw materials such as agricultural waste, manure, municipal waste, plant material, sewage, green waste, Wastewater treatment, wastewater, and food waste. Biogas is produced by anaerobic ...
, from industrial processes or via
direct air-captured carbon dioxide) via
biomethanation, where biomethanogens (archaea) consume carbon dioxide and hydrogen and produce methane within an
anaerobic
Anaerobic means "living, active, occurring, or existing in the absence of free oxygen", as opposed to aerobic which means "living, active, or occurring only in the presence of oxygen." Anaerobic may also refer to:
*Adhesive#Anaerobic, Anaerobic ad ...
environment. This process is highly efficient, as the archaea are self-replicating and only require low-grade (60 °C) heat to perform the reaction.
Another process has also been achieved by
SoCalGas to convert the carbon dioxide in raw biogas to methane in a single electrochemical step, representing a simpler method of converting excess renewable electricity into storable natural gas.
The UK has completed surveys and is preparing to start injecting hydrogen into the gas grid as the grid previously carried 'town gas' which is a 50% hydrogen-methane gas formed from coal. Auditors KPMG found that converting the UK to hydrogen gas could be £150bn to £200bn cheaper than rewiring British homes to use electric heating powered by lower-carbon sources.
Excess power or off peak power generated by wind generators or solar arrays can then be used for load balancing in the energy grid. Using the existing natural gas system for hydrogen, Fuel cell maker
Hydrogenics and natural gas distributor
Enbridge
Enbridge Inc. is a multinational pipeline transport, pipeline and energy company headquartered in Calgary, Alberta, Canada. Enbridge owns and operates pipelines throughout Canada and the United States, transporting crude oil, natural gas, and n ...
have teamed up to develop such a
power to gas system in Canada.
where a natural gas network is used for the storage of hydrogen. Before switching to
natural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
, the German gas networks were operated using
towngas, which for the most part (60-65%) consisted of hydrogen. The storage capacity of the German natural gas network is more than 200,000 GW·h which is enough for several months of energy requirement. By comparison, the capacity of all German pumped storage power plants amounts to only about 40 GW·h. The transport of energy through a gas network is done with much less loss (<0.1%) than in a power network (8%). The use of the existing
natural gas pipelines for hydrogen was studied by NaturalHy.
Automotive onboard hydrogen storage
Portability is one of the biggest challenges in the
automotive industry
The automotive industry comprises a wide range of company, companies and organizations involved in the design, Business development, development, manufacturing, marketing, selling, Maintenance, repairing, and Custom car, modification of motor ve ...
, where high density storage systems are problematic due to safety concerns.
High-pressure tanks weigh much more than the hydrogen they can hold. For example, in the 2014
Toyota Mirai, a full tank contains only 5.7% hydrogen, the rest of the weight being the tank.
System densities are often around half those of the working material, thus while a material may store 6
wt% H
2, a working system using that material may only achieve 3 wt% when the weight of tanks, temperature and pressure control equipment, etc., is considered.
Fuel cells and storage
Due to its clean-burning characteristics, hydrogen is a clean fuel alternative for the automotive industry. Hydrogen-based fuel could significantly reduce the emissions of
greenhouse gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. T ...
es such as CO
2, SO
2 and NO
x. Three problems for the use of
hydrogen fuel cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requ ...
(HFC) are efficiency, size, and safe onboard storage of the gas. Other major disadvantages of this emerging technology involve cost, operability and durability issues, which still need to be improved from the existing systems. To address these challenges, the use of nanomaterials has been proposed as an alternative option to the traditional hydrogen storage systems. The use of nanomaterials could provide a higher density system and increase the driving range towards the target set by the
DOE at 300 miles. Carbonaceous materials such as
carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range ( nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
* ''Single-walled carbon nanotubes'' (''S ...
and metal hydrides are the main focus of research. They are currently being considered for onboard storage systems due to their versatility, multi-functionality, mechanical properties and low cost with respect to other alternatives.
Other advantages of nanomaterials in fuel cells
The introduction of nanomaterials in onboard hydrogen storage systems may be a major turning point in the automotive industry. However, storage is not the only aspect of the fuel cell to which nanomaterials may contribute. Different studies have shown that the transport and
catalytic properties of
Nafion
Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer-copolymer synthesized in 1962 by Dr. Donald J. Connolly at the DuPont Experimental Station in Wilmington Delaware (U.S. Patent 3,282,875). Additional work on the polym ...
membranes used in
HFCs can be enhanced with
TiO2/
SnO2 nanoparticles.
The increased performance is caused by an improvement in hydrogen splitting
kinetics due to
catalytic activity of the nanoparticles. Furthermore, this system exhibits faster transport of
proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s across the cell which makes
HFCs with nanoparticle composite membranes a promising alternative.
Another application of nanomaterials in water splitting has been introduced by a research group at
Manchester Metropolitan University
Manchester Metropolitan University is located in the centre of Manchester, England. The university has 40,000 students and over 4,000 members of staff. It is home to four faculties (Arts and Humanities, Business and Law, Health and Education ...
in the UK using screen-printed
electrodes
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety ...
consisting of a
graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
-like material. Similar systems have been developed using
photoelectrochemical techniques.
Pressurized hydrogen gas
Increasing gas pressure improves the energy density by volume making for smaller container tanks. The standard material for holding pressurised hydrogen in tube trailers is steel (there is no
hydrogen embrittlement
Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can Permeation, permeate solid metals. O ...
problem with hydrogen gas). Tanks made of carbon and glass fibres reinforcing plastic as fitted in Toyota Mirai and Kenworth trucks are required to meet safety standards. Few materials are suitable for tanks as hydrogen being a small molecule tends to diffuse through many polymeric materials. The most common on board hydrogen storage in 2020 vehicles was hydrogen at pressure 700bar = 70MPa. The energy cost of compressing hydrogen to this pressure is significant.
Pressurized gas pipelines are always made of steel and operate at much lower pressures than tube trailers.
Liquid hydrogen
Alternatively, higher volumetric energy density liquid hydrogen or
slush hydrogen Slush hydrogen is a combination of liquid hydrogen and solid hydrogen at the triple point with a lower temperature and a higher density than liquid hydrogen. It is commonly formed by repeating a freeze-thaw process. This is most easily done by brin ...
may be used. However, liquid hydrogen is cryogenic and boils at 20.268 K (−252.882 °C or −423.188 °F).
Cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th International Institute of Refrigeration's (IIR) International Congress of Refrigeration (held in Washington, DC in 1971) endorsed a univers ...
storage cuts weight but requires large
liquification
In materials science, liquefaction is a process that generates a liquid from a solid or a gas or that generates a non-liquid phase which behaves in accordance with fluid dynamics.
It occurs both naturally and artificially. As an example of t ...
energies. The liquefaction process, involving pressurizing and cooling steps, is energy intensive.
The liquefied hydrogen has lower energy density by volume than gasoline by approximately a factor of four, because of the low density of liquid hydrogen – there are actually more oxidizable hydrogen atoms in a litre of gasoline (116 grams) than there are in a litre of pure liquid hydrogen (71 grams). Like any other liquid at
cryogenic temperatures, the liquid hydrogen storage tanks must also be well insulated to minimize boil off.
Japan has a liquid hydrogen (LH2) storage facility at a terminal in Kobe, and was expected to receive the first shipment of liquid hydrogen via LH2 carrier in 2020. Hydrogen is liquified by reducing its temperature to −253 °C, similar to liquified natural gas (LNG) which is stored at −162 °C. A potential efficiency loss of 12.79% can be achieved, or 4.26 kWh/kg out of 33.3 kWh/kg.
Liquid organic hydrogen carriers (LOHC)
Research
The Hydrogen Storage Materials research field is vast, having tens of thousands of published papers. According to Papers in the 2000 to 2015 period collected from Web of Science and processed in VantagePoint bibliometric software, a scientometric review of research in hydrogen storage materials was constituted. According to the literature, hydrogen energy went through a hype-cycle type of development in the 2000s. Research in Hydrogen Storage Materials grew at increasing rates from 2000 to 2010. Afterwards, growth continued but at decreasing rates, and a plateau was reached in 2015. Looking at individual country output, there is a division between countries that after 2010 inflected to a constant or slightly declining production, such as the European Union countries, the US and Japan, and those whose production continued growing until 2015, such as China and South Korea. The countries with most publications were China, the EU and the United States, followed by Japan. China kept the leading position throughout the entire period, and had a higher share of hydrogen storage materials publications in its total research output.
Among materials classes, Metal-Organic Frameworks were the most researched materials, followed by Simple Hydrides. Three typical behaviors were identified:
# New materials, researched mainly after 2004, such as MOFs and Borohydrides;
# Classic materials, present through the entire period with growing number of papers, such as Simple Hydrides, and
# Materials with stagnant or declining research through the end of the period, such as AB5 alloys and Carbon Nanotubes.
However, current
physisorption
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely wikt:perturb, perturbed upon adsorption.
Overview
The fundamental interacting force of physisorption is Van der Waals ...
technologies are still far from being commercialized. The experimental studies are executed for small samples less than 100 g. The described technologies require high pressure and/or low temperatures as a rule. Therefore, at their current state of the art these techniques are not considered as a separate novel technology but as a type of valuable add-on to current compression and
liquefaction
In materials science, liquefaction is a process that generates a liquid from a solid or a gas or that generates a non-liquid phase which behaves in accordance with fluid dynamics.
It occurs both naturally and artificially. As an example of t ...
methods.
Physisorption processes are reversible since no activation energy is involved and the interaction energy is very low. In materials such as
metal–organic framework
Metal–organic frameworks (MOFs) are a class of porous polymers consisting of metal cluster compound, clusters (also known as Secondary Building Units - SBUs) coordinated to organic compound, organic ligands to form one-, two- or three-dimension ...
s, porous carbons, zeolites, clathrates, and organic polymers, hydrogen is physisorbed on the surface of the pores. In these classes of materials, the hydrogen storage capacity mainly depends on the surface area and pore volume. The main limitation of use of these sorbents as H
2storage materials is weak van der Waals interaction energy between hydrogen and the surface of the sorbents. Therefore, many of the physisorption based materials have high storage capacities at liquid nitrogen temperature and high pressures, but their capacities become very low at ambient temperature and pressure.
LOHC, liquid organic hydrogen storage systems is a promising technique for future hydrogen storage. LOHC are
organic compounds
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
that can absorb and release
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
through
chemical reactions
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products ...
. These compounds are characterized by the fact that they can be loaded and un-loaded with considerable amounts of hydrogen in a cyclic process. In principle, every unsaturated compound (organic molecules with C-C
double
Double, The Double or Dubble may refer to:
Mathematics and computing
* Multiplication by 2
* Double precision, a floating-point representation of numbers that is typically 64 bits in length
* A double number of the form x+yj, where j^2=+1
* A ...
or
triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, sin ...
s) can take up hydrogen during
hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
. This technique ensures that the release of compounds into the atmosphere are entirely avoided in hydrogen storage. Therefore, LOHCs is an attractive way to provide wind and solar energy for mobility applications in the form of liquid energy carrying molecules of similar energy storage densities and manageability as today's fossil fuels.
See also
*
Cascade storage system
*
Cryo-adsorption
*
Electrochemical hydrogen compressor An electrochemical hydrogen compressor is a hydrogen compressor where hydrogen is supplied to the anode, and compressed hydrogen is collected at the cathode with an exergy efficiency up to and even beyond 80% for pressures up to 10,000 psi or 700 b ...
*
Hydrogenography
*
Hydrogen energy plant in Denmark
*
Industrial gas
Industrial gases are the gaseous materials that are Manufacturing, manufactured for use in Industrial sector, industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other ...
*
Tunable nanoporous carbon
*
Combined cycle hydrogen power plant
*
Grid energy storage
Grid energy storage, also known as large-scale energy storage, are technologies connected to the electrical power grid that store energy for later use. These systems help balance supply and demand by storing excess electricity from variabl ...
*
Hydrogen infrastructure
*
Hydrogen economy
The hydrogen economy is an umbrella term for the roles hydrogen can play alongside low-carbon electricity to reduce emissions of greenhouse gases. The aim is to reduce emissions where cheaper and more energy-efficient clean solutions are not ava ...
*
Hydrogen turboexpander-generator A hydrogen turboexpander-generator or generator-loaded expander for hydrogen gas is an axial flow turbine or Turboexpander, radial expander for energy recovery through which a high pressure hydrogen gas is expanded to produce work used to drive an e ...
*
Power-to-gas
*
Timeline of hydrogen technologies
This is a timeline of the history of hydrogen technology.
Timeline
16th century
* c. 1520 – First recorded observation of hydrogen by Paracelsus through dissolution of metals (iron, zinc, and tin) in sulfuric acid.
17th century
* 1625 – F ...
References
External links
Hydrogen Supply Availability with Cavern StorageLarge Hydrogen Underground StorageWasserstoff-Speicherung in Salzkavernen zur Glättung des Windstromangebots
(German)
1993-Energy and hydrogen Pag.482009-SNL-Geologic Storage of Hydrogen
Hydrogen stored in salt caverns could be converted into flexible power sourcearchive
MaHyTec Hydrogen Tanks
*
Hydrogen as the fuel of the future, report by the DLR; discusses the types of hydrogen storage
Ammonia Borane (NhxBHx)
* Research into metal–organic framework or Nano Cage
H2 Storage Projects
{{DEFAULTSORT:Hydrogen Storage
Hydrogen storage,
Sustainable technologies
Energy storage
Industrial gases
Gas technologies

 Chemical storage could offer high storage performance due to the high storage densities. For example, supercritical hydrogen at 30 °C and 500 bar only has a density of 15.0 mol/L while
Chemical storage could offer high storage performance due to the high storage densities. For example, supercritical hydrogen at 30 °C and 500 bar only has a density of 15.0 mol/L while