Trace metal stable isotope biogeochemistry is the study of the distribution and relative abundances of trace metal
isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s in order to better understand the biological, geological, and chemical processes occurring in an environment.
Trace metal
Trace metals are the metals subset of trace elements; that is, metals normally present in small but measurable amounts in animal and plant cells and tissues. Some of these trace metals are a necessary part of nutrition and physiology. Some bi ...
s are elements such as
iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
,
magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
,
copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
, and
zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
that occur at low levels in the environment. Trace metals are critically important in biology and are involved in many processes that allow organisms to grow and generate energy. In addition, trace metals are constituents of numerous rocks and minerals, thus serving as an important component of the geosphere. Both
stable
A stable is a building in which working animals are kept, especially horses or oxen. The building is usually divided into stalls, and may include storage for equipment and feed.
Styles
There are many different types of stables in use tod ...
and
radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
isotopes of trace metals exist, but this article focuses on those that are stable. Isotopic variations of trace metals in samples are used as
isotopic fingerprints to elucidate the processes occurring in an environment and answer questions relating to biology, geochemistry, and medicine.
Isotope notation
In order to study trace metal stable isotope biogeochemistry, it is necessary to compare the relative abundances of isotopes of trace metals in a given biological, geological, or chemical pool to a standard (discussed individually for each isotope system below) and monitor how those relative abundances change as a result of various biogeochemical processes. Conventional notations used to mathematically describe isotope abundances, as exemplified here for
56Fe, include the isotope ratio (
56R), fractional abundance (
56F) and delta notation (δ
56Fe). Furthermore, as different biogeochemical processes vary the relative abundances of the isotopes of a given trace metal, different reaction pools or substances will become enriched or depleted in specific isotopes. This partial separation of isotopes between different pools is termed
isotope fractionation
Isotope fractionation describes fractionation processes that affect the relative abundance of isotopes, a phenomena that occurs (and so advantage is taken of it) in the study geochemistry, biochemistry, food science, and other fields. Normally, ...
, and is mathematically described by fractionation factors α or ε (which express the difference in isotope ratio between two pools), or by "cap delta" (Δ; the difference between two δ values). For a more complete description of these notations, see the isotope notation section in
Hydrogen isotope biogeochemistry
Hydrogen isotope biogeochemistry (HIBGC) is the scientific study of biological, geological, and chemical processes in the environment using the distribution and relative abundance of hydrogen isotopes. Hydrogen has two stable isotopes, protium H an ...
.
Naturally occurring trace metal isotope variations and fractionations
In nature, variations in isotopic ratios of trace metals on the order of a few tenths to several ‰ are observed within and across diverse environments spanning the geosphere, hydrosphere and biosphere. A complete understanding of all processes that fractionate trace metal isotopes is presently lacking, but in general, isotopes of trace metals are fractionated during various chemical and biological processes due to
kinetic
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to its motion
Art and ente ...
and
equilibrium isotope effects.
Geochemical fractionations
Certain isotopes of trace metals are preferentially oxidized or reduced; thus, transitions between redox species of the metal ions (e.g., Fe
2+ → Fe
3+) are fractionating, resulting in different isotopic compositions between the different redox pools in the environment.
Additionally, at high temperatures, metals ions can
evaporate
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when hum ...
(and subsequently
condense
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
upon cooling), and the relative differences in isotope masses of a given heavy metal leads to fractionation during these evaporation and condensation processes.
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
of isotopes through a solution or material can also result in fractionations, as the lighter mass isotopes are able to diffuse at a faster rate.
Additionally, isotopes can have slight variations in their solubility and other chemical and physical properties, which can also drive fractionation.
Biological fractionations
In sediments, oceans, and rivers, distinct trace metal isotope ratios exist due to biological processes such as metal ion uptake and abiotic processes such as adsorption to particulate matter that preferentially remove certain isotopes.
The trace metal isotopic composition of a given organism results from a combination of the isotopic compositions of source material (i.e., food and water) and any fractionations imparted during metal ion uptake, translocation and processing inside cells.
Applications of trace metal isotope ratios
Stable isotope ratios of trace metals can be used to answer a variety of questions spanning diverse fields, including oceanography, geochemistry, biology, medicine, anthropology and astronomy. In addition to their modern applications, trace metal isotopic compositions can provide insight into ancient biogeochemical processes operated on Earth.
These signatures arise because the processes that form and modify samples are recorded in the trace metal isotopic compositions of the samples. By analyzing and understanding trace metal isotopic compositions in biological, chemical or geological materials, one can answer questions such as the sources of nutrients for phytoplankton in the ocean, processes that drove the formation of geologic structures, the diets of modern or ancient organisms, and accretionary processes that took place in the early Solar System.
Trace metal stable isotope biogeochemistry is still an emerging field, yet each trace metal isotope system has clear, powerful applications to diverse and important questions. Important heavy metal isotope systems are discussed (in order of increasing atomic mass) in the proceeding sections.
Iron
Stable isotopes and natural abundances
Naturally occurring
iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
has four stable
isotopes
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but ...
,
54Fe,
56Fe,
57Fe, and
58Fe.
Stable iron isotopes are described as the relative abundance of each of the stable isotopes with respect to
54Fe. The standard for iron is elemental iron, IRMM-014, and it is distributed by the Institute for Reference Materials and Measurement. The delta value is compared to this standard, and is defined as:
Delta values are often reported as per mil values (‰), or part-per-thousand differences from the standard. Iron isotopic fractionation is also commonly described in units of per mil per atomic mass unit.
In many cases, the δ
56Fe value can be related to the δ
57Fe and δ
58Fe values through mass-dependent fractionation:
Chemistry
One of the most prevalent features of iron chemistry is its
redox chemistry
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
. Iron has three
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s: metallic iron (Fe
0), ferrous iron (Fe
2+), and ferric iron (Fe
3+). Ferrous iron is the reduced form of iron, and ferric iron is the oxidized form of iron. In the presence of oxygen, ferrous iron is oxidized to ferric iron, thus ferric iron is the dominant redox state of iron at Earth's surface conditions. However, ferrous iron is the dominant redox state below the surface at depth. Because of this redox chemistry, iron can act as either an electron donor or receptor, making it a metabolically useful species.
Each form of iron has a specific distribution of electrons (i.e.,
electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
), tabulated below:
Equilibrium Isotope Fractionation
Variations in iron isotopes are caused by a number of chemical processes which result in the preferential incorporation of certain isotopes of iron into certain phases. Many of the chemical processes which fractionate iron are not well understood and are still being studied. The most well-documented chemical processes which fractionate iron isotopes relate to its redox chemistry, the evaporation and condensation of iron, and the diffusion of dissolved iron through systems. These processes are described in more detail below.
Fractionation as a result of redox chemistry
To first order, reduced iron favors isotopically light iron and oxidized iron favors isotopically heavy iron. This effect has been studied in regards to the abiotic oxidation of Fe
2+ to Fe
3+, which results in fractionation. The mineral
ferrihydrite
Ferrihydrite (Fh) is a widespread hydrous ferric oxyhydroxide mineral at the Earth's surface, and a likely constituent in extraterrestrial materials. It forms in several types of environments, from freshwater to marine systems, aquifers to hydro ...
, which forms in acidic aquatic conditions, is precipitated via the oxidation of aqueous Fe
2+ to Fe
3+.
Precipitated ferrihydrite has been found to be enriched in the heavy isotopes by 0.45‰ per atomic mass unit with respect to the starting material.
This indicates that heavier iron isotopes are preferentially precipitated as a result of oxidizing processes.
Theoretical calculations in combination with experimental data have also aimed to quantify the fractionation between Fe(III)
aq and Fe(II)
aq in HCl.
Based on modeling, the fractionation factor between the two species is temperature dependent:
Fractionation as a result of evaporation and condensation
Evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evapora ...
and
condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
can give rise to both kinetic and equilibrium isotope effects. While equilibrium mass fractionation is present evaporation and condensation, it is negligible compared to kinetic effects.
During condensation, the condensate is enriched in the light isotope, whereas in evaporation, the gas phase is enriched in the light isotope. Using the
kinetic theory of gases
The kinetic theory of gases is a simple classical model of the thermodynamic behavior of gases. Its introduction allowed many principal concepts of thermodynamics to be established. It treats a gas as composed of numerous particles, too small ...
, for
56Fe/
54Fe, a fractionation factor of α = 1.01835 for the evaporation of a pool containing equimolar amounts of
56Fe and
54Fe.
In evaporation experiments, the evaporation of FeO at 1,823 K gave a fractionation factor of α = 1.01877.
Presently, there have been no experimental attempts to determine the
56Fe/
54Fe fractionation factor of condensation.
Fractionation as a result of diffusion
Kinetic fractionation of dissolved iron occurs as a result of
diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
. When isotopes diffuse, the lower mass isotopes diffuse more quickly than the heavier isotopes, resulting in fractionation. This difference in diffusion rates has been approximated as:
In this equation, D
1 and D
2 are the diffusivities of the isotopes, m
1 and m
2 are the masses of the isotopes, and β, which can vary between 0 and 0.5, depending on the system.
More work is required to fully understand fractionation as a result of diffusion, studies of diffusion of iron on metal have consistently given β values of approximately 0.25. Iron diffusion between silicate melts and basaltic/rhyolitic melts have given lower β values (~0.030). In aqueous environments, a β value of 0.0025 has been obtained.
Fractionation as a result of phase partitioning
There may be equilibrium fractionation between coexisting minerals. This would be particularly relevant when considering the formation of planetary bodies early in the
Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Sola ...
. Experiments have aimed to simulate the formation of the Earth at high temperatures using a platinum-iron alloy and an analog for the silicate earth at 1,500 °C.
However, the observed fractionation was very small, less than 0.2‰ per atomic mass unit.
More experimental work is needed to fully understand this effect.
Biology
In biology, iron plays a number of roles. Iron is widespread in most living organisms and is essential for their function. In microbes, iron redox chemistry is utilized as an electron donor or receptor in microbial metabolism, allowing microbes to generate energy. In the oceans, iron is essential for the growth and survival of phytoplankton, which use iron to fix nitrogen. Iron is also important in plants, given that they need iron to transfer electrons during photosynthesis. Finally, in animals, iron plays many roles, however, its most essential function is to transport oxygen in the bloodstream throughout the body. Thus, iron undergoes many biological processes, each of which have variations in which isotopes of iron they preferentially use. While iron isotopic fractionations are observed in many organisms, they are still not well understood. Improvements in the understanding the iron isotope fractionations observed in biology will enable the development of a more complete knowledge of the enzymatic, metabolic, and other biologic pathways in different organisms. Below, the known iron isotopic variations for different classes of organisms are described.
Iron reducing bacteria
Iron reducing bacteria reduce ferric iron to ferrous iron under anaerobic conditions. One of the first studies that studied iron fractionation in iron-reducing bacteria studied the bacterium ''
Shewanella algae''.
''S. algae'' was grown on a
ferrihydrite
Ferrihydrite (Fh) is a widespread hydrous ferric oxyhydroxide mineral at the Earth's surface, and a likely constituent in extraterrestrial materials. It forms in several types of environments, from freshwater to marine systems, aquifers to hydro ...
substrate, and was then allowed to reduce iron.
The study found that ''S. algae'' preferentially reduced
54Fe over
56Fe, with a δ
56/54Fe value of -1.3‰.
More recent experiments have studied the bacterium ''Shewanella putrefaciens'' and its reduction of Fe(III) in
goethite
Goethite (, ) is a mineral of the diaspore group, consisting of iron(III) oxide-hydroxide, specifically the α- polymorph. It is found in soil and other low-temperature environments such as sediment. Goethite has been well known since ancient t ...
. These studies have found δ
56/54Fe values of -1.2‰ relative to the goethite.
The kinetics of this fractionation were also studied in this experiment, and it was suggested that the iron isotope fractionation is likely related to the kinetics of the
electron transfer
Electron transfer (ET) occurs when an electron relocates from an atom, ion, or molecule, to another such chemical entity. ET describes the mechanism by which electrons are transferred in redox reactions.
Electrochemical processes are ET reactio ...
step.
Most studies of other iron reducing bacteria have found δ
56/54Fe values of approximately -1.3‰.
At high Fe(III) reduction rates, δ
56/54Fe values of -2 – -3‰ relative to the substrate have been observed.
The study of iron isotopes in iron reducing bacteria enable the development of an improved understanding regarding the metabolic processes operating in these organisms.
Iron oxidizing bacteria
While most iron is oxidized as a result of interaction with atmospheric oxygen or oxygenated waters, oxidation by bacteria is an active process in anoxic environments and in oxygenated, low pH (<3) environments. Studies of the
acidophilic
Acidophiles or acidophilic organisms are those that thrive under highly acidic conditions (usually at pH 5.0 or below). These organisms can be found in different branches of the tree of life, including Archaea, Bacteria,Becker, A.Types of Bacteri ...
Fe(II)-oxidizing bacterium, ''
Acidthiobacillus ferrooxidans'', have been used to determine the fractionation as a result of iron-oxidizing bacteria. In most cases, δ
56/54Fe values between 2 and 3‰ were measured.
However, a Rayleigh trend with a fractionation factor of α
Fe(III)aq-Fe(II)aq ~ 1.0022 was observed, which is smaller than the fractionation factor in the abiotic control experiments (α
Fe(III)aq-Fe(II)aq ~ 1.0034), which has been inferred to reflect a biological isotope effect.
Using iron isotopes, an improvement in the understanding of the metabolic processes controlling iron oxidation and energy production in these organisms can be developed.
Photoautrophic bacteria, which oxidize Fe(II) under
anaerobic
Anaerobic means "living, active, occurring, or existing in the absence of free oxygen", as opposed to aerobic which means "living, active, or occurring only in the presence of oxygen." Anaerobic may also refer to:
*Adhesive#Anaerobic, Anaerobic ad ...
conditions, have also been studied. The ''Thiodictyon'' bacteria precipitate poorly crystalline hydrous ferric oxide when they oxidize iron.
The precipitate was enriched in the
56Fe relative to Fe(II)
aq, with a δ
56/54Fe value of +1.5 ± 0.2‰.
Magnetotactic bacteria
Magnetotactic bacteria
Magnetotactic bacteria (or MTB) are a polyphyletic group of bacteria that orient themselves along the magnetic field lines of Earth's magnetic field. Discovered in 1963 by Salvatore Bellini and rediscovered in 1975 by Richard Blakemore, this alig ...
are bacteria with
magnetosome
Magnetosomes are membranous structures present in magnetotactic bacteria (MTB). They contain iron-rich magnetic particles that are enclosed within a lipid bilayer membrane. Each magnetosome can often contain 15 to 20 magnetite crystals that form a ...
s that contain magnetic crystals, usually
magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
or
greigite
Greigite is an iron sulfide mineral with the chemical formula . It is the sulfur equivalent of the iron oxide magnetite (Fe3O4). It was first described in 1964 for an occurrence in San Bernardino County, California, and named after the mineral ...
, which allow them to orient themselves with the Earth’s
magnetic field lines
A magnetic field (sometimes called B-field) is a physical field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular ...
. These bacteria mineralize magnetite via the reduction of Fe(III), usually in microaerobic or anoxic environments. In the magnetotactic bacteria that have been studied, there was no significant iron isotope fractionation observed.
Phytoplankton
Iron is important for the growth of
phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
. In phytoplankton, iron is used for electron transfer reactions in
photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
in both
photosystem I
Photosystem I (PSI, or plastocyanin–ferredoxin oxidoreductase) is one of two photosystems in the Light-dependent reactions, photosynthetic light reactions of algae, plants, and cyanobacteria. Photosystem I is an integral membrane ...
and
photosystem II
Photosystem II (or water-plastoquinone oxidoreductase) is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem ...
.
Additionally, iron is an important component of the enzyme
nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only fa ...
, which is used to fix nitrogen.
In measurements at open ocean stations, phytoplankton are isotopically light, with the fractionation as a result of biological uptake measured between -0.25‰ and -0.13‰. Improvement in the understanding of this fractionation will enable the more precise understanding of phytoplankton photosynthetic processes.
Animals
Iron has many important roles in animal biology, specifically when considering oxygen transport in the bloodstream, oxygen storage in muscles, and enzymes. Known isotope variations are shown in the figure below. Iron isotopes could be useful tracers of the iron biochemical pathways in animals, and also be indicative of
trophic level
The trophic level of an organism is the position it occupies in a food web. Within a food web, a food chain is a succession of organisms that eat other organisms and may, in turn, be eaten themselves. The trophic level of an organism is the ...
s in a food chain.
Iron isotope variations in humans reflects a number of processes. Specifically, iron in the blood stream reflects dietary iron, which is isotopically lighter than iron in the geosphere.
Iron isotopes are distributed heterogeneously throughout the body, primarily to red blood cells, the liver, muscle, skin, enzymes, nails, and hair. Iron losses in the body (intestinal bleeding, bile, sweat, etc.) favor the loss of isotopically heavy iron, with mean losses averaging a δ
56Fe of +10‰.
Iron absorption in the intestine favors lighter iron isotopes.
This is largely due to the fact that iron is carried by
transport proteins
A transport protein (variously referred to as a transmembrane pump, transporter, escort protein, acid transport protein, cation transport protein, or anion transport protein) is a protein that serves the function of moving other materials within ...
and
transferrin
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Iron(III), Fe3+ ions. Human transferrin is ...
, both of which are kinetic processes, resulting in the preferential uptake of isotopically light iron.
The observed iron isotopic variations in humans and animals are particularly important as tracers. Iron isotopic signatures are utilized to determine the geographic origin of food. Additionally, anthropologists and paleontologists use iron isotope data in order to track the transfer of iron between the geosphere and the biosphere, specifically between plant foods and animals. This allows for the reconstruction of ancient dietary habits based on the variations in iron isotopes in food.

Geochemistry
By mass, iron is the most common element on Earth, and it is the fourth most abundant element in the Earth's
crust. Thus, iron is widespread throughout the geosphere, and is also common on other planetary bodies. Natural variations in the iron in the geosphere are relatively small. Currently, the values of δ
56/54Fe measured in rocks and minerals range from -2.5‰ to +1.5‰. Iron isotope composition is homogeneous in igneous rocks to ±0.05‰, indicating that much of the geologic isotopic variability is a result of the formation of rocks and minerals at low temperature.
This homogeneity is particularly useful when tracing processes which result in fractionation through the system. While fractionation of igneous rocks is relatively constant, there are larger variations in the iron isotopic composition of chemical sediments.
Thus, iron isotopes are used to determine the origin of the protolith of heavily metamorphosed rocks of a sedimentary origin.
Improvements of the understanding regarding the way in which iron isotopes fractionate in the geosphere can help to better understand geologic processes of formation.
Natural iron isotopic variations
To date, iron is one of the most widely studied trace metals, and iron isotope compositions are relatively well-documented. Based on measurements, iron isotopes exhibit minimal variation (±3‰) in the terrestrial environment. A list of iron isotopic values of different materials from different environments is presented below.

In terrestrial environments
There is an extreme constancy of the isotopic composition of igneous rocks. The mean value of δ
56Fe of terrestrial rocks is 0.00 ± 0.05‰.
More precise isotopic measurements indicate that the small deviations from 0.00‰ may reflect a slight mass-dependent fractionation.
This mass fractionation has been proposed to be F
Fe = 0.039 ± 0.008‰ per atomic mass unit relative to IRMM-014.
There may also be slight isotopic variations in igneous rocks depending on their composition and process of formation. The average value of δ
56Fe for ultramafic igneous rocks is -0.06‰, whereas the average value of δ
56Fe for mid-ocean ridge basalts (MORB) is +0.03‰.
Sedimentary rocks exhibit slightly larger variations in δ
56Fe, with values between -1.6‰ and +0.9‰ relative to IRMM-014.
Banded iron formations δ
56Fe span the entire range observed on Earth, from -2.5‰ to +1‰.
In the oceans
There are slight iron isotopic variations in the oceans relative to IRMM-014, which likely reflect variations in the biogeochemical cycling of iron within a given ocean basin. In the southeastern Atlantic, δ
56Fe values between -0.13 and +0.21‰ have been measured. In the north Atlantic, δ
56Fe values between -1.35 and +0.80‰ have been measured. In the equatorial Pacific δ
56Fe values between -0.03 and +0.58‰ have been measured. The supply of aerosol iron particles to the ocean have an isotopic composition of approximately 0‰.
Dissolved iron riverine input to the ocean is isotopically light relative to igneous rocks, with δ
56Fe values between -1 and 0‰.
Most modern marine sediments have δ
56Fe values similar to those of igneous δ
56Fe values.
Marine
ferromanganese nodules
Ferromanganese is an alloy of iron and manganese, with other elements such as silicon, carbon, sulfur, nitrogen and phosphorus. The primary use of ferromanganese is as a type of processed manganese source to add to different types of steel, such ...
have δ
56Fe values between -0.8 and 0‰.
In hydrothermal systems
Hot (> 300 °C)
hydrothermal
Hydrothermal circulation in its most general sense is the circulation of hot water (Ancient Greek ὕδωρ, ''water'',Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon. revised and augmented throughout by Sir Henry Stuart Jones. with th ...
fluids from mid ocean ridges are isotopically light, with δ
56Fe between -0.2 and -0.8‰.
Particles in hydrothermal plumes are isotopically heavy relative to the hydrothermal fluids, with δ
56Fe between 0.1 and 1.1‰.
Hydrothermal deposits have average δ
56Fe between -1.6 and 0.3‰.
The
sulfide mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide () as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenide mineral, selenides, the tell ...
s within these deposits have δ
56Fe between -2.0 and 1.1‰.
In extraterrestrial objects
Variations in iron isotopic composition have been observed in meteorite samples from other planetary bodies. The
Moon
The Moon is Earth's only natural satellite. It Orbit of the Moon, orbits around Earth at Lunar distance, an average distance of (; about 30 times Earth diameter, Earth's diameter). The Moon rotation, rotates, with a rotation period (lunar ...
has variations in iron isotopes of 0.4‰ per atomic mass unit.
Mars
Mars is the fourth planet from the Sun. It is also known as the "Red Planet", because of its orange-red appearance. Mars is a desert-like rocky planet with a tenuous carbon dioxide () atmosphere. At the average surface level the atmosph ...
has very small isotope fractionation of 0.001 ± 0.006‰ per atomic mass unit.
has iron fractionations of 0.010 ± 0.010‰ per atomic mass unit.
The
chondritic reservoir exhibits fractionations of 0.069 ± 0.010‰ per atomic mass unit.
Isotopic variations observed on planetary bodies can help to constrain and better understand their formation and processes occurring in the early Solar System.
Measurement
High precision iron isotope measurements are obtained either via
thermal ionization mass spectrometry
Thermal ionization mass spectrometry (TIMS), also known as surface ionization, is a highly sensitive isotope mass spectrometry characterization technique. The isotopic ratios of radionuclides are used to get an accurate measurement for the elemen ...
(TIMS) or multi-collector
inductively coupled plasma mass spectrometry
Inductively coupled plasma mass spectrometry (ICP-MS) is a type of mass spectrometry that uses an inductively coupled plasma to ionize the sample. It atomizes the sample and creates atomic and small polyatomic ions, which are then detected. It i ...
(MC-ICP-MS).
Applications of iron isotopes
Iron isotopes have many applications in the geosciences, biology, medicine, and other fields. Their ability to act as isotopic tracers allows for their use to determine information regarding the formation of geologic units and as a potential proxy for life on Earth and other planets. Iron isotopes also have applications in anthropology and paleontology, as they are used to study the diets of ancient civilizations and animals. The widespread uses of iron in biology make its isotopes a promising frontier in biomedical research, specifically their use to prevent and treat blood conditions and other pathological blood diseases. Some of the more prevalent applications of iron isotopes are described below.
Banded iron formations
Banded iron formation
Banded iron formations (BIFs; also called banded ironstone formations) are distinctive units of sedimentary rock consisting of alternating layers of iron oxides and iron-poor chert. They can be up to several hundred meters in thickness and e ...
s (BIFs) are particularly important when considering the surface environments of the early Earth, which were significantly different from the surface environments observed today. This is manifested in the mineralogy of these formations, which are indicative of different redox conditions.
Additionally, BIFs are interesting in that they were deposited while major changes were occurring in the atmosphere and in the biosphere 2.8 to 1.8 billion years ago.
Iron isotopic studies can reveal the details of the formation of BIFs, which allows for the reconstruction of redox and climatic conditions at the time of deposition.
BIFs formed as a result of the oxidation of iron by oxygen, which was likely generated by the evolution of
cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteri ...
.
This was followed by the subsequent precipitation of iron particles in the ocean.
Observed variations in the iron isotopic composition of BIFs span the entire range observed on Earth, with δ
56/54Fe values between -2.5 and +1‰.
The cause of these variations are hypothesized to occur for three reasons. The first relates to the varying mineralogy of the BIFs. Within the BIFs, minerals such as
hematite
Hematite (), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of . ...
,
magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
,
siderite
Siderite is a mineral composed of iron(II) carbonate (FeCO3). Its name comes from the Ancient Greek word (), meaning "iron". A valuable iron ore, it consists of 48% iron and lacks sulfur and phosphorus. Zinc, magnesium, and manganese commonly ...
, and
pyrite
The mineral pyrite ( ), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue ...
are observed.
These minerals each having varying isotopic fractionation, likely as a result of their structures and the kinetics of their growth.
The isotopic composition of the BIFs is indicative of the fluids from which they precipitated, which has applications when reconstructing environmental conditions of the ancient Earth.
It has also been suggested that BIFs may be biologic in origin. The range of their δ
56/54Fe values fall within the range of those observed to occur as a result of biologic processes relating to bacterial metabolic processes, such as those of anoxygenic phototrophic iron-oxidizing bacteria.
Ultimately, the improved understanding of BIFs using iron isotope fractionations would allow for the reconstruction of past environments and the constraint of processes occurring on the ancient Earth. However, given that the values observed as a result of biogenic and abiogenic fractionation are relatively similar, the exact processes of BIFs are still unclear. Thus, the continued study and improved understanding of biologic and abiologic fractionation effects would be beneficial in providing better details regarding BIF formation.
Iron cycling in the ocean

Iron isotopes have become particularly useful in recent years for tracing biogeochemical cycling in the oceans. Iron is an important
micronutrient
Micronutrients are essential chemicals required by organisms in small quantities to perform various biogeochemical processes and regulate physiological functions of cells and organs. By enabling these processes, micronutrients support the heal ...
for living species in the ocean, particularly for the growth of phytoplankton. Iron is estimated to limit phytoplankton growth in about one half of the ocean.
As a result, the development of a better understanding of sources and cycling of iron in the modern oceans is important. Iron isotopes have been used to better constrain these pathways through data collected by the
GEOTRACES
GEOTRACES is an international research programme for improving understanding of marine biogeochemical cycles.
GEOTRACES is organised internationally under the auspices of the Scientific Committee on Oceanic Research (originally under the Int ...
program, which has collected iron isotopic data throughout the ocean. Based on the variations in iron isotopes, biogeochemical cycling and other processes controlling iron distribution in the ocean can be elucidated.
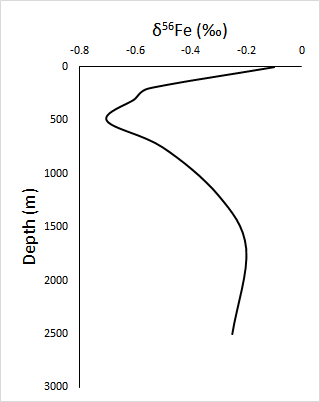
For example, the combination of iron concentration and iron isotope data can use to determine the sources of oceanic iron. In the South Atlantic and in the Southern Ocean, isotopically light iron is observed in intermediate waters (200 - 1,300 meters), whereas isotopically heavy iron is observed in surface waters and deep waters (> 1,300 meters).
To first order, this demonstrates that there are different sources, sinks, and processes contributing to the iron cycle in varying water masses. The isotopically light iron in intermediate waters suggests that the dominant iron sources include remineralized organic matter.
This organic matter is isotopically light because phytoplankton preferentially take up light iron.
In the surface ocean, the isotopically heavy iron represents the external sources of iron, such as dust, which is isotopically heavy relative to IRMM-014, and the sink of light isotopes as a result of their preferential uptake by phytoplankton.
The isotopically heavy iron in the deep ocean suggests that the iron cycle is dominated by the abiotic, non-reductive release of iron, via
desorption
Desorption is the physical process where Adsorption, adsorbed atoms or molecules are released from a surface into the surrounding vacuum or fluid. This occurs when a molecule gains enough energy to overcome the activation barrier and the binding e ...
or
dissolution, from particles.
Isotopic analyses similar to the one above are utilized throughout all of the world's oceans to better understand regional variability in the processes which control iron cycling. These analyses can then be synthesized to better model the global biogeochemical cycling of iron, which is particularly important when considering primary production in the ocean.
Constraining processes on extraterrestrial bodies
Iron isotopes have been applied for a number of purposes on planetary bodies. Their variations have been measured to more precisely determine the processes that occurred during
planetary accretion
In astrophysics, accretion is the accumulation of particles into a massive object by gravitationally attracting more matter, typically gaseous matter, into an accretion disk. Most astronomical objects, such as galaxies, stars, and planets, are fo ...
. In the future, the comparison of observed biological fractionation of iron on Earth to fractionation on other planetary bodies may have
astrobiological
Astrobiology (also xenology or exobiology) is a scientific field within the List of life sciences, life and environmental sciences that studies the abiogenesis, origins, Protocell, early evolution, distribution, and future of life in the univ ...
implications.
= Planetary accretion
=
One of the primary challenges in the study of planetary accretion is the fact that many tracers of the processes occurring in the early Solar System have been eliminated as a result of subsequent geologic events. Because transition metals do not show large stable isotope fractionations as a result of these events and because iron is one of the most abundant elements in the terrestrial planets, its isotopic variability has been used as a tracer of early Solar System processes.
Variations in δ
57/54Fe between samples from
Vesta,
Mars
Mars is the fourth planet from the Sun. It is also known as the "Red Planet", because of its orange-red appearance. Mars is a desert-like rocky planet with a tenuous carbon dioxide () atmosphere. At the average surface level the atmosph ...
, the
Moon
The Moon is Earth's only natural satellite. It Orbit of the Moon, orbits around Earth at Lunar distance, an average distance of (; about 30 times Earth diameter, Earth's diameter). The Moon rotation, rotates, with a rotation period (lunar ...
, and Earth have been observed, and these variations cannot be explained by any known petrological, geochemical, or planetary processes, thus, it has been inferred that the observed fractionations are a result of planetary accretion.
It is interesting to note that the isotopic compositions of the Earth and the Moon are much heavier than that of Vesta and Mars. This provides strong support for the
giant-impact hypothesis
The giant-impact hypothesis, sometimes called the Theia Impact, is an astrogeology hypothesis for the formation of the Moon first proposed in 1946 by Canadian geologist Reginald Daly. The hypothesis suggests that the Early Earth collided wi ...
as an impact of this energy would generate large amounts of energy, which would melt and vaporize iron, leading to the preferential escape of the lighter iron isotopes to space.
More of the heavier isotopes would remain, resulting in the heavier iron isotopic compositions observed for the Earth and the Moon. The samples from Vesta and Mars exhibit minimal fractionation, consistent with the theory of runaway growth for their formations, as this process would not yield significant fractionations.
Further study of the stable isotope of iron in other planetary bodies and samples could provide further evidence and more precise constraints for planetary accretion and other processes that occurred in the early Solar System.
= Astrobiology
=
The use of iron isotopes may also have applications when studying potential evidence for life on other planets. The ability of microbes to utilize iron in their metabolisms makes it possible for organisms to survive in anoxic, iron-rich environments, such as Mars. Thus, the continual improvement of knowledge regarding the biological fractionations of iron observed on Earth can have applications when studying extraterrestrial samples in the future. While this field of research is still developing, this could provide evidence regarding whether a sample was generated as a result of biologic or abiologic processes depending on the isotopic fractionation. For example, it has been hypothesized that magnetite crystals found in
Martian meteorite
A Martian meteorite is a rock that formed on Mars, was ejected from the planet by an impact event, and traversed interplanetary space before landing on Earth as a meteorite. , 277 meteorites had been classified as Martian, less than half a perce ...
s may have formed biologically as a result of their striking similarity to magnetite crystals produced by magnetotactic bacteria on Earth.
Iron isotopes could be used to study the origin of the proposed "
magnetofossils" and other rock formations on Mars.
Biomedical research
Iron plays many roles in human biology, specifically in
oxygen transport, short-term
oxygen storage Methods of oxygen storage for subsequent use span many approaches, including high pressures in oxygen tanks, cryogenics, oxygen-rich compounds and reaction mixtures, and chemical compounds that reversibly release oxygen upon heating or pressure chan ...
, and
metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
Iron also plays a role in the body's
immune system
The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to bacteria, as well as Tumor immunology, cancer cells, Parasitic worm, parasitic ...
.
Current biomedical research aims to use iron isotopes to better understand the speciation of iron in the body, with hopes of eventually being able to reduce the availability of free iron, as this would help to defend against infection.
Iron isotopes can also be utilized to better understand iron absorption in humans.
The iron isotopic composition of blood reflects an individual's long-term absorption of dietary iron.
This allows for the study of genetic predisposition to blood conditions, such as
anemia
Anemia (also spelt anaemia in British English) is a blood disorder in which the blood has a reduced ability to carry oxygen. This can be due to a lower than normal number of red blood cells, a reduction in the amount of hemoglobin availabl ...
, which will ultimately enable the prevention, identification, and resolution of blood disorders.
Iron isotopic data could also aid in identifying impairments of the iron absorption regulatory system in the body, which would help to prevent the development of pathological conditions related to issues with iron regulation.
Cobalt
Nickel
Copper
Stable isotopes and natural abundances
Copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
has two naturally occurring stable
isotopes
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but ...
:
63Cu and
65Cu, which exist in the following natural abundances:
The isotopic composition of Cu is conventionally reported in delta notation (in ‰) relative to a NIST SRM 976 standard:


 Iron isotopes have become particularly useful in recent years for tracing biogeochemical cycling in the oceans. Iron is an important
Iron isotopes have become particularly useful in recent years for tracing biogeochemical cycling in the oceans. Iron is an important 